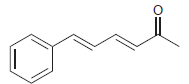
Question: Use resonance structures to help you identify all sites of low electron density (δ+) in the following compound:
Use resonance structures to help you identify all sites of low electron density (δ+) in the following compound:
Step by Step Solution
★★★★★
3.42 Rating (168 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

5... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


