Addition of HC1 to 1-methoxycyclohexene yields 1-chloro-1-methoxycyclo- hexane as the sole product. Use resonance structures to explain
Question:
Addition of HC1 to 1-methoxycyclohexene yields 1-chloro-1-methoxycyclo- hexane as the sole product. Use resonance structures to explain why none of the other regioisomer isformed.
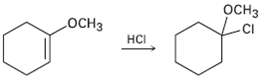
Transcribed Image Text:
оснз оСнз HCI CI CI
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 27% (11 reviews)
ficos OCH 3 H OCH3 Conjugation with the oxygen lone pair electrons makes the double bond more nucl...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Addition of HC1 to 1, 2-dimethylcyclohexene yields a mixture of two products. Show the stereochemistry of each, and explain why a mixture is formed.
-
Use resonance structures to help you identify all sites of low electron density (δ+) in the following compound:
-
Use resonance formulas to explain why polyacetylene has delocalized molecular orbitals extending over the length of the molecule, whereas the following molecule does not. HHHHH
-
Pipestone Ltd. (Pipestone) uses an aging schedule to estimate the amount of receivables that won't be collected. Pipestone allows its customers up to 60 days to pay amounts owed. Any receivable...
-
Why can't car dealerships simply keep paying based on past systems?
-
Let Compute the indicated matrices (if possible). 2D - 5A 2 1 C = 3 |A = 4 B = -2 3 4 2 3 5 5 6 -3 E = [4 2], F= D = %3D 2.
-
Describe three ways of preventing confl ict.
-
Everyone needs an emergency fund. Assume your best friend asks you to evaluate a list of investments for an emergency savings fund. Comment on the appropriateness of each of the following: a....
-
Pierce Black is a working at a law firm. His salary recently increased and he would like to keep track of his net worth Pierce has gathered the following information. Assume his net worth at the...
-
Marquita Filters produces an air filter for use in jet aircraft. Parts are added at several points in the production process. In August, production began with 600 filters in Work in Process, 80...
-
Myrcene, C10H16, is found in oil of bay leaves and is isomeric with ?-Ocimene (Problem 14.48). It has an ultraviolet absorption at 226 nm and can be catalytically hydrogenated to yield 2,...
-
Hydrocarbon A, C 10 H 14 , has a UV absorption at ? max = 236 nm and gives hydrocarbon B, C 10 H 18 , on catalytic hydrogenation. Ozonolysis of A followed by zinc/acetic acid treatment yields the...
-
The Nebraska Public Power District (NPPD) operated the Cooper Nuclear Station and instituted a "fitness for duty" program that required all employees who had access to protected areas at the Cooper...
-
What could a team leader do to determine whether individuals or teams require extra support?
-
Write a MATLAB script to visualize a parametric surface representing a torus ( doughnut shape ) ?in 3 D space. The parametric equations for a torus with major radius R and minor radius r are given...
-
Read the synopsis just above or next to the video clip, then view the clip in its entirety. here is the link https://broadwayeconomics.com/gaston/ https://broadwayeconomics.com/gaston/. (In some...
-
On your 23rd birthday you decide to invest $4,500 (10% of your annual salary) in a mutual fund earning 7% per year. You will continue to make annual deposits equal to 10% of your annual salary until...
-
The graph of a function f is given. Sketch the graphs of the following transformations of f. y 5 -4 -2 2 4 6 5 00 8 10 10 x
-
When would the researcher choose to use partial least squares structural equation modeling (PLS-SEM)?
-
The age-old saying for investing is "buy low and sell high," but this is easier said than done. Investors who panic about falling prices sell their investments, which in turn lowers the price and...
-
A solution contains 22.4 g glucose (C 6 H 12 O 6 ) dissolved in 0.500 L of water. What is the molality of the solution? (Assume a density of 1.00 g/mL for water.) a) 0.238 m b) 44.8 m c) 0.249 m d)...
-
Which of the following compounds would give a positive Tollens test? (Remember that the Tollens test involves mild basic aqueous conditions.) (a) CH3CH2CH2COCH3 (b) CH3CH2CH2CH2CHO (c)...
-
Solving the following road-map problem depends on determining the structure of A, the key intermediate. Give structures for compounds A through K. hept-1-yne SOCI CH3CuLi (2) (CHj,S (2) Ho HCN (2)...
-
The UV spectrum of an unknown compound shows values of λmax at 225 nm (ε = 10,000) and at 318 nm 1e = 402. The mass spectrum shows a molecular ion at m/z 96 and a prominent...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


