Use resonance structures to help you identify all sites of high electron density (δ-) in the following
Question:
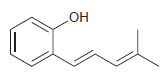
Transcribed Image Text:
Он
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)

Answered By

Sandip Nandnawar
I am a B.E (Information technology) from GECA and also have an M.C.M from The University of RTMNU, MH.
I worked as a software developer (Programmer and TL). Also working as an expert for the last 6 years and deal with complex assessment and projects. I have a team and lead a team of experts and conducted primary and secondary research. I am a senior software engg and senior expert and deal with all types of CSE and IT and other IT-related assessments and projects and homework.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Use resonance structures to help you identify all sites of low electron density (δ+) in the following compound:
-
Phenol, C6H5OH, is a stronger acid then methanol, CH3OH, even though both contains an O ? H bond. Draw the structures of the anions resulting from loss of H+ from phenol and methanol, and use...
-
Addition of HC1 to 1-methoxycyclohexene yields 1-chloro-1-methoxycyclo- hexane as the sole product. Use resonance structures to explain why none of the other regioisomer isformed. HCI CI CI
-
Determine whether the given set of matrices under the specified operation, matrix addition or multiplication, is a group. Recall that a diagonal matrix is a square matrix whose only nonzero entries...
-
Verna's makes all sales on account, subject to the following collection pattern: 25% are collected in the month of sale; 60% are collected in the first month after sale; and 15% are collected in the...
-
Use a triple integral to find the volume of the solid bounded by the graphs of the equations. z = 9 - x, y = -x + 2, y = 0, z = 0, x 0
-
The second most named woman was Sarah Palin, who was named by 15% of the people in the sample. Use a technology tool to find a 95% confidence interval for the population propor- tion that would have...
-
Prepare general journal entries for the following transactions of Sustain Company. Use the following (partial) chart of accounts: Cash; Prepaid Insurance; Accounts Receivable; Furniture; Accounts...
-
Bryant Co. reports net income of $20,000. For the year, depreciation expense is $7.000 and the company reports a gain of $3.000 from sale of machinery. It also had a $2,000 loss from retirement of...
-
Zaids financial year ends on 31 October. On 1 November 206 he owed advertising costs of $40. During the year ended 31 October 207 he paid advertising costs of $530. This included $270 for an...
-
Calculate the volume of all gases evolved by the complete oxidation of 0.375 g of the amino acid alanine NH 2 CHCH 3 COOH if the products are liquid water, nitrogen gas, and carbon dioxide gas, the...
-
As a result of photosynthesis, an acre of forest (1 acre = 4047 square meter) can take up 1000. kg of CO 2 . Assuming air is 0.0314% CO 2 by volume, what volume of air is required to provide 350. kg...
-
Steam-driven power generators rotate at a constant speed via a governor that maintains constant steam pressure in the turbine. In addition, automatic generation control (AGC) or load frequency...
-
Section Three Answer the questions below 1.While pulling out of her driveway, Bethany becomes distracted by a bee and strikes Melanie, who is riding past on a bicycle. Bethany suffers serious injury...
-
A __________ is a schedule periodic check of a specific process behavior. Question 1Answer A. Widget B. Dashboard C. Monitor D. Process ID
-
1. Was VAAF contractually obligated to pay Chad for refraining from smoking? 2. Was there consideration to support its promise to pay $500? 3. Are there other facts you need to know to make that...
-
Presented here are the comparative balance sheets of Hames Incorporated at December 31, 2023 and 2022. Sales for the year ended December 31, 2023, totaled $1,700,000.%0D%0A%0D%0AHAMES...
-
McDonald's conducts operations worldwide and is managed in two primary geographic segments: US, and International Operated Markets, which is comprised of Australia, Canada, France, Germany, Italy,...
-
Solve each system using the elimination method. If a system is inconsistent or has dependent equations, say so. X =x + 1 || 2 || = 2 00/ 8 5 5
-
a. Why does the Wi-Fi Alliance release compatibility testing profiles in waves instead of combining the entire standards features initially? 27a1.) An 802.11ac Wi-Fi compatibility testing profile...
-
Ricinoleic acid, a compound that can be isolated from castor oil, has the structure CH3(CH2)5CHOHCH2CH==CH(CH2)7CO2H. (a) How many stereoisomers of this structure are possible? (b) Write these...
-
There are two dicarboxylic acids with the general formula HO2CCH==CHCO2H. One dicarboxylic acid is called maleic acid; the other is called fumaric acid. When treated with OsO4, followed by...
-
Use your answers to the preceding problem to predict the stereochemical outcome of the addition of bromine to maleic acid and to fumaric acid. (a) Which dicarboxylic acid would add bromine to yield a...
-
Management makes many judgements and estimates in preparing accounts, some of which will have a significant effect on the reported results and financial position. Give examples of ZAIN estimates and...
-
What is the NPV of a project with an initial investment of $350,000 and annual cash inflows of $150,000 for the next 10 years? Cost of capital is 13% A $436,721.21 B $442,901.59 C $452,932.43 D...
-
Journal DATE DESCRIPTION POST. REF. DEBIT CREDIT 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Joumalize the entries for the following transactions. Refer to the Chart of Accounts for exact wording of...

Study smarter with the SolutionInn App


