Stability of compounds in new drugs. Refer to the ACS Medicinal Chemistry Letters (Vol. 1, 2010) study
Question:
Stability of compounds in new drugs. Refer to the ACS Medicinal Chemistry Letters (Vol. 1, 2010) study of the metabolic stability of drugs, Exercise 2.35
(p. 77). Recall that two important values computed from the testing phase are the fraction of compound unbound to plasma (fup) and the fraction of compound unbound to microsomes (fumic). A key formula for assessing stability assumes that the fup/fumic ratio is 1. Pharmacologists at Pfizer Global Research and Development tested 416 drugs and reported the fup/fumic ratio for each. These data are saved in the FUP file, and summary statistics are provided in the accompanying MINITAB printout. Suppose the pharmacologists want to determine if the true mean ratio, m, differs from 1.
a. Specify the null and alternative hypothesis for this test.
b. Descriptive statistics for the sample ratios are provided in the accompanying MINITAB printout. Note that the sample mean, x = .327, is less than 1. Consequently, a pharmacologist wants to reject the null hypothesis.
What are the problems with using such a decision rule?
c. Locate values of the test statistic and corresponding p-value on the printout.
d. Select a value of
a, the probability of a Type I error.
Interpret this value in the words of the problem.
e. Give the appropriate conclusion, based on the results of parts c and d.
f. What conditions must be satisfied for the test results to be valid?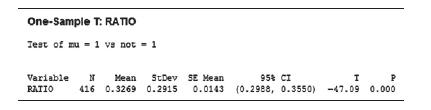
Step by Step Answer:

Statistics Plus New Mylab Statistics With Pearson Etext Access Card Package
ISBN: 978-0134090436
13th Edition
Authors: James Mcclave ,Terry Sincich





