Stability of compounds in new drugs. Testing the metabolic stability of compounds used in drugs is the
Question:
Stability of compounds in new drugs. Testing the metabolic stability of compounds used in drugs is the cornerstone of new drug discovery. Two important values computed from the testing phase are the fraction of compound unbound to plasma (fup) and the fraction of compound unbound to microsomes (fumic). A key formula for assessing stability assumes that the fup/fumic ratio is 1. Pharmacologists at Pfizer Global Research and Development investigated this phenomenon and reported the results in ACS Medicinal Chemistry Letters (Vol. 1, 2010). The fup/fumic ratio was determined for each of 416 drugs in the Pfizer database. A graph describing the fup/
fumic ratios is shown below.
a. What type of graph is displayed?
b. What is the quantitative variable summarized in the graph?
c. Determine the proportion of fup/fumic ratios that fall above 1.
d. Determine the proportion of fup/fumic ratios that fall below .4.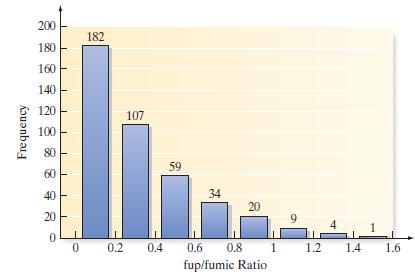
Step by Step Answer:

Statistics Plus New Mylab Statistics With Pearson Etext Access Card Package
ISBN: 978-0134090436
13th Edition
Authors: James Mcclave ,Terry Sincich




