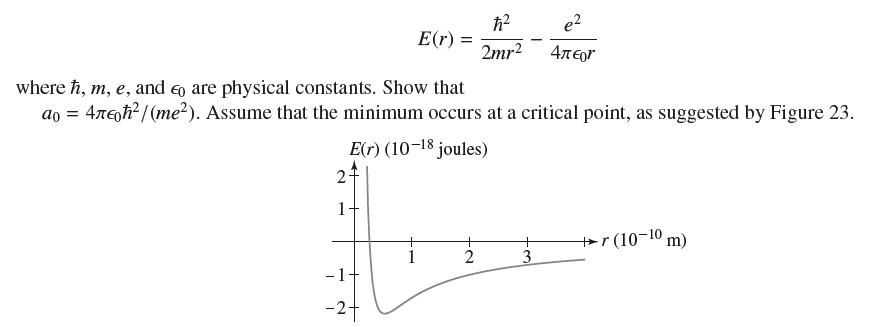
The Bohr radius a 0 of the hydrogen atom is the value of r that minimizes the
Question:
The Bohr radius a0 of the hydrogen atom is the value of r that minimizes the energy
Transcribed Image Text:
2 1- E(r) = where ħ, m, e, and o are physical constants. Show that ao = 4лεħ²/(me²). Assume that the minimum occurs at a critical point, as suggested by Figure 23. E(r) (10-18 joules) - 1 -24 ħ² 2mr² 2 + e² 4πeor 3 +r (10-10 m)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Let Then impli...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
The hydrogen atom is composed of one proton in the nucleus and one electron, which moves about the nucleus. In the quantum theory of atomic structure, it is assumed that the electron does not move in...
-
The ground-state wave function of a hydrogen atom is = (1/xa3)1/2e-r1ao where ao = 53 pm (the Bohr radius). (a) Calculate the probability that the electron will be found somewhere within a small...
-
The wave function for the 2pz orbital in the hydrogen atom is Where a0 is the value for the radius of the first Bohr orbit in meters (5.29 Ã 10-11), Ï is Zr/a0, r is the value for the...
-
Two wires carrying equal and opposite currents are twisted together in the construction of a circuit. Why does this technique reduce stray magnetic fields? Please explain for dummies.
-
A corporation issued $1,000,000 of common stock in exchange for $1,000,000 of fixed assets. Where would this transaction be reported on the statement of cash flows?
-
A truck with the capacity to load 2,200 cubic feet of cargo is available to transport items selected from the following table. If selected, an item must be shipped in its entirety (i.e., partial...
-
Q8 What are the tasks for system maintenance?
-
The following comparative income statement (in thousands of dollars) for two recent years was adapted from the annual report of Speedway Motorsports, Inc., owner and operator of several major motor...
-
Which of the following statements is false? Select one: a. the normal balance of cash account is debit balance b. the normal balance of unearned revenue of credit balance c. the normal balance of...
-
Spotlight Ltd has issued share capital of 60,000----8% redeemable cumulative preference shares of ~ 20 each and 4,00,000 equity shares of ~ 10 each. The preference shares are redeemable at a premium...
-
The response of a circuit or other oscillatory system to an input of frequency (omega) is described by the function 50 () = @ (A) D = 0.01 Both wo (the natural frequency of the system) and D (the...
-
Prove that x = 4 is the greatest root of (x) = x 4 8x 2 128.
-
In Problems 712, determine whether the given sequence is arithmetic, geometric, or neither. If the sequence is arithmetic, find the common difference and the sum of the first n terms. If the sequence...
-
Discuss how technology and human resources are needed to operate this facility in this behind the scenes look at this retailing giant. Support your opinion with research and/or key concepts covered...
-
4.2 At a given instant, a spacecraft is 500 km above the earth, with a right ascension of 300 and a declination of -20 relative to the geocentric equatorial frame. Its velocity is 10 km/s directly...
-
A 447 gram cart (mA) slides along a very smooth track and collides with a stationary 475 gram cart (mB). A motion detector records the velocity of cart A, as shown in Figures 1 and 2. A force probe...
-
M8 Homework i Saved 1 Mayfair Company completed the following transactions and uses a perpetual inventory system. Help Save & Exit Submit Check my work 10 points eBook Print References June 4 Sold...
-
Free Response Table Problem x -6 -80 -4 -3 f(x) 1.948 1 0 -2 -2.005 -798 undefined -2 -1.995 0 1 1.995 2 2.005 6 80 802 4 3.333 3.001 undefined 2.998 2.5 2.048 23. The table above represents values...
-
The life spans of wild tribbles are normally distributed with a mean value of 1.8 years and a standard deviation of 0.8 year. Sketch the normal curve, and shade the portion of the graph showing...
-
Teasdale Inc. manufactures and sells commercial and residential security equipment. The comparative unclassified balance sheets for December 31, 2015 and 2014 are provided below. Selected missing...
-
Which function approaches 0 faster as x approaches infinity: e-x or 1/x? Many improper integrals can be evaluated by comparing functions with the method of leading behavior. State which of the given...
-
Evaluate the following improper integrals or say why they don't converge. dx 0 (2+5x
-
Use the comparison test to deduce whether the following improper integrals converge. If they do, find an upper bound on the value. dx
-
Mak Incredint Corp. on My 1, 2017 for $1. The per conocer Shoe On this dates, Sink had common shers outstanding of $65,000 and retained endng of $80,0. The onderden ble ethely to pow. Selected sounds...
-
On www.arey Octave 46.000.000 for 30.000.000.000.00.000 of owered and in 2000.000 barres ofreced and Theo Walther will be removed one between 2007 ON 0.000 OLVO CG OD WM On www.arey Octave 46.000.000...
-
2.1 Patrick Ntuli, a dealer in property, on 26 February, agreed to sell a country estate (a property) to his out of wedlock child, Venecia Thames, for its market value of R2 000 000. Patrick Ntuli...

Study smarter with the SolutionInn App


