The methane molecule CH4 consists of a carbon molecule bonded to four hydrogen molecules that are spaced
Question:
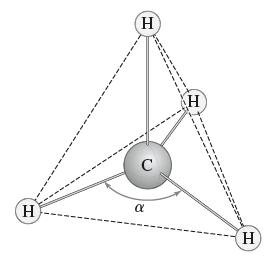
The methane molecule CH4 consists of a carbon molecule bonded to four hydrogen molecules that are spaced as far apart from each other as possible. The hydrogen atoms then sit at the vertices of a tetrahedron, with the carbon atom at its center, as in Figure 22. We can model this with the carbon atom at the point (1/2 , 1/2 , 1/2) and the hydrogen atoms at (0, 0, 0), (1, 1, 0), (1, 0, 1), and (0, 1, 1). Use the dot product to find the bond angle α formed between any two of the line segments from the carbon atom to the hydrogen atoms.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





