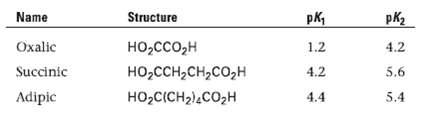
Shown here are some pKa data for simple dibasic acids. How can you account for the fact
Question:
Shown here are some pKa data for simple dibasic acids. How can you account for the fact that the difference between the first and second ionization constants decreases with increasing distance between the carboxyl groups?
Transcribed Image Text:
Name Structure pk, pK2 Oxalic Succinic 4.2 1.2 HO,cco,H но -ссн-сн,со,н но -СICH2),cо,H 4.2 5.6 4.4 Adipic 5.4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
Inductive effects of functional groups are transmitted through bonds For oxalic acid the e...View the full answer

Answered By

SUMAN DINDA
I LIKE TO TEACH STUDENTS. SO, I START MYSELF AS A PRIVATE TUTOR. I TEACH STUDENTS OF DIFFERENT CLASSES. I HAVE ALSO DONE BACHELOR OF EDUCATION DEGREE(B.ED). DURING THIS COURSE I HAD TO TEACH IN A SCHOOL. SO I HAVE A GOOD EXPERIENCE IN TEACHING.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How can you account for the fact that 2, 2, 6-trimethylcyclohexanone yields no detectable aldol product even though it has an acidic hydrogen?
-
How can you account for the fact that cis-1, 3-pentadicne is much less reactive than trans-1, 3-pentadiene in the DielsAlder reaction?
-
How can you account for the fact that normal rain is slightly acidic?
-
The following data applies to the two unrelated companies Lloyd Ltd and Cole Ltd: All taxable and deductible temporary differences relate to the profit or loss. Assume a corporate tax rate of 30%. A....
-
1. To what degree do you agree or disagree with the B-Team's goal for world governments to reach net zero CO2 or greenhouse gas (GHG) emissions by 2050? 2. Should governments of developing economies...
-
Based on the answers to Exercises 7.89 to 7.91, what factors have an impact on a researchers ability to reject the null hypothesis? Which one(s) can he or she control?
-
Do you agree with corporates decision that Theo is an excellent candidate for this new position? What about his background and experience would help prepare him to succeed at the Lincoln Hotel? Why...
-
For the current year, Bearings Company decided to switch from the indirect method to the direct method for reporting cash flows from operating activities on the statement of cash flows. Will the...
-
You have $25,000 to invest in a stock portfolio. Your choices are Stock X with an expected return of 13 percent and Stock Y with an expected return of 8 percent. (a) If your goal is to create a...
-
After Enron, WorldCom, and other major corporate scandals that rocked America in the recent past, it seemed that nothing would surprise investors or regulators. However, almost everyone was shocked...
-
In humans, the final product of purine degradation from DNA is uric acid, pKa = 5.61, which is excreted in the urine. What is the percent dissociation of uric acid in urine at a typical pH = 6.0? Why...
-
Predict the product of the reaction of p-methyl benzoic acid with each of the following: (a) LiAlH 4 , then H 3 O + (b) N-Bromosuccinimide in CCl 4 (c) CH 3 MgBr in ether, then H 3 O + (d) KMnO 4 , H...
-
Using the example of an online cell phone apps store, list relevant data flows, data stores, processes, and sources/sinks. Draw a context diagram and a level-0 diagram that represent the apps store....
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,500 cases of Oktoberfest-style beer from a German supplier for 390,000 euros. Relevant U.S. dollar exchange rates for the euro...
-
Palmerstown Company established a subsidiary in a foreign country on January 1, Year 1, by investing 8,000,000 pounds when the exchange rate was $1.00/pound. Palmerstown negotiated a bank loan of...
-
Required information [The following information applies to the questions displayed below.] The following is financial information describing the six operating segments that make up Chucktown Sauce...
-
Question 1 (50 marks) Costa Ltd is a company with a 30 June year end. The following information relates to Costa Ltd and its subsidiary Jumbo for the year ended 30 June 20.22. Costa Ltd Jumbo Ltd Dr...
-
The following salaried employees of Mountain Stone Brewery in Fort Collins, Colorado, are paid semimonthly. Some employees have union dues or garnishments deducted from their pay. You do not need to...
-
Many of the employees of East Coast Yachts have shares of stock in the company because of an existing employee stock purchase plan. To sell the stock, the employees can tender their shares to be sold...
-
By referring to Figure 13.18, determine the mass of each of the following salts required to form a saturated solution in 250 g of water at 30 oC: (a) KClO3, (b) Pb(NO3)2, (c) Ce2(SO4)3.
-
What are the name and formula of the compound commonly used in the manufacture of glass to increase the index of refraction?
-
Treating oxytocin with certain reducing agents (e.g., sodium in liquid ammonia) brings about a single chemical change that can be reversed by air oxidation. What chemical changes are involved?
-
What classes of reactions are involved in the cleavage of the Fmoc group with piperidine, leading to the unprotected amino acid and the fluorene by-product? Write mechanisms for these reactions.
-
Show all steps in the synthesis of GVA using the tert-butyloxycarbonyl (Boc) group as a protecting group.
-
20 On January 1, Year 1, X Company purchased equipment for $80,000. The company estimates that the equipment will have a useful life of 10 years and a residual value of $5,000. X Company depreciates...
-
Discuss why it is important for company managers to understand and use social capital knowledge to help build social ties among their skilled knowledge workers so they can build employee loyalty...
-
Kate lives in a house close to a local university, and she traditionally has rented a garage apartment in the back of her property to students for $750 per month. Kate wants to transfer the title to...

Study smarter with the SolutionInn App


