a. What downward transitions are possible for a sodium atom in the 6s state? (See Figure 41.24.)
Question:
a. What downward transitions are possible for a sodium atom in the 6s state? (See Figure 41.24.)
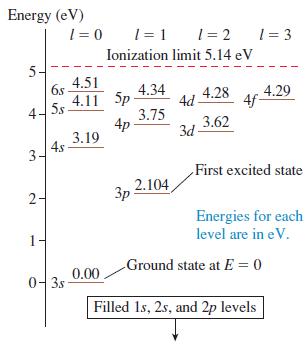
b. What are the wavelengths of the photons emitted in each of these transitions?
Transcribed Image Text:
Energy (eV) 1 = 0 1 = 1 1 = 2 1 = 3 Ionization limit 5.14 eV 5- 4.51 4.34 4.11 5p 3.75 4p 3.19 6s 4.28 4.29 4d 4f- 5s 4 3.62 3d 4s 3- First excited state 2.104 3p Energies for each level are in eV. 1 Ground state at E = 0 0-3s 0.00 Filled Is, 2s, and 2p levels 2.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
Visualize Solve a The allowed transitions are those with l ...View the full answer

Answered By

Khurram shahzad
I am an experienced tutor and have more than 7 years’ experience in the field of tutoring. My areas of expertise are Technology, statistics tasks I also tutor in Social Sciences, Humanities, Marketing, Project Management, Geology, Earth Sciences, Life Sciences, Computer Sciences, Physics, Psychology, Law Engineering, Media Studies, IR and many others.
I have been writing blogs, Tech news article, and listicles for American and UK based websites.
4.90+
5+ Reviews
17+ Question Solved
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Mathematics questions
-
A sodium atom (Z = 11) contains 11 protons in its nucleus. Strictly speaking, the Bohr model does not apply, because the neutral atom contains 11 electrons instead of a single electron. However, we...
-
What are the wavelengths of the two photons produced when a proton and anti proton at rest annihilate?
-
What are the wavelengths of the two photons produced when a proton and an antiproton at rest annihilate?
-
Discuss the two-pipe system, how it works, and its advantages and disadvantages.
-
A mass of 1.5 kg of air at 120 kPa and 24C is contained in a gas-tight, frictionless piston cylinder device. The air is now compressed to a final pressure of 600 kPa. During the process, heat is...
-
Beach Company reports the following data: Sales Variable costs Fixed costs $800.000 300.000 220.000 Beach Company's operating leverage is fround to one decimal) 0 2.7 O 1.8 1.3 6.7
-
Bad habits. According to the National Household Survey on Drug Abuse, 40.4% of adolescents aged 12 to 17 years used alcohol in 2006, 17.3% used marijuana, 2.2% used cocaine, and 25.8% used...
-
The records of Grade A Steak Company list the following selected accounts for the quarter ended April 30, 2015: Requirements 1. Prepare a single-step income statement. 2. Prepare a multi- step income...
-
Barney Googal owns a garage and is contemplating purchasing a tire retreading machine for $26,820. After estimating costs and revenues, Barney projects a net cash inflow from the retreading machine...
-
Which of the graphs in Fig. Q25.12 best illustrates the current I in a real resistor as a function of the potential difference V across it? Explain. Figure Q25.12 (a) (b) (c) (d)
-
The 5d 3p transition in the emission spectrum of sodium has a wavelength of 499 nm. What is the energy of the 5d state?
-
Draw a series of pictures, similar to Figure 41.22, for the ground states of Ca, Ni, As, and Kr. 2p 2s %23 %23 1s Z = 5 B 1s25 2p Z = 6 C 1s 2s2p? Z =7 N 1s 2s2p Z = 8 0 1s2s2p* Z = 9 F 1s 2s2p Z =...
-
Jana Morgan is about to sign up for cellular telephone service. She is primarily interested in the safety aspect of the phone; that is, she wants to have one available for emergencies. She does not...
-
Identify a public conflict (such as a recent Congressional debate or even a celebrity breakup) that has come to the forefront in the media (or public's attention) in the last thirty days. You have...
-
Performance Management Issues You have been asked to return to your alma mater and speak to current students about performance management issues. To make the most of this experience for yourself and...
-
Analysis of competitor organization of our selected organization Walmart and its competitor Safeway. 1. Complete analysis of competitor organization; addresses all relevant factors and typically uses...
-
Defining Program Objectives of Youth centers Clearly define the objectives of your program or center. What specific outcomes do you hope to achieve? Examples may include promoting physical fitness,...
-
Identify a local or regional organization and analyze how they demonstrate servant leadership in their operations. You will want to review their website, social media, news, and other resources to...
-
Alternative Flour Company manufactures flour by a series of three processes, beginning in the Milling Department. From the Milling Department, the materials pass through the Sifting and Packaging...
-
Based on the scenario described below, generate all possible association rules with values for confidence, support (for dependent), and lift. Submit your solutions in a Word document (name it...
-
Ann (mass 50 kg) is standing at the left end of a 15-m-long, 500 kg cart that has frictionless wheels and rolls on a frictionless track. Initially both Ann and the cart are at rest. Suddenly, Ann...
-
Force F x = (10 N) sin(2t/4.0 s) is exerted on a 250 g particle during the interval 0s t 2.0s. If the particle starts from rest, what is its speed at t = 2.0 s?
-
Force F x = (10 N) sin(2t/4.0 s) is exerted on a 250 g particle during the interval 0s t 2.0s. If the particle starts from rest, what is its speed at t = 2.0 s?
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


