Question: The hydrogen atom 1s wave function is a maximum at r = 0. But the 1s radial probability density, shown in Figure 41.8, peaks at
The hydrogen atom 1s wave function is a maximum at r = 0. But the 1s radial probability density, shown in Figure 41.8, peaks at r = aB and is zero at r = 0. Explain this paradox.
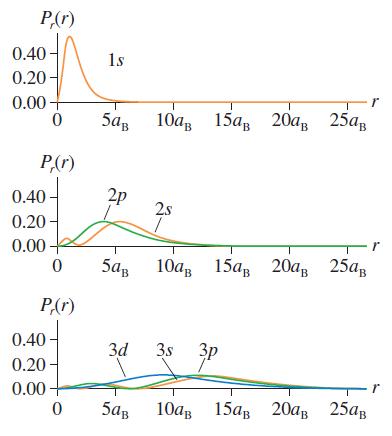
P(r) 0.40- 0.20- 1s 0.00 10, 15, 20, 25, P,(r) 2p 0.40 0.20 2s 0.00 5ag 10 15, 20, 25 P(r) 0.40 3d 3s 3p 0.20 0.00 5aB 10 15 20 25
Step by Step Solution
3.31 Rating (163 Votes )
There are 3 Steps involved in it
The radial probability density P r r 4r 2 R nl r is the probability density for fi... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
2071_60b9d844d8253_856114.pdf
180 KBs PDF File
2071_60b9d844d8253_856114.docx
120 KBs Word File


