Find the general solution for each differential equation. Verify that each solution satisfies the original differential equation.
Question:
Find the general solution for each differential equation. Verify that each solution satisfies the original differential equation.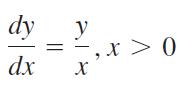
Transcribed Image Text:
dy y = dx X 0 < x
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
dy dx X dy dx S ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
Find the general solution for each differential equation. dy x + 2y - ex = 0 dx
-
Find the general solution for each differential equation. x ln x dy dx + y = 2x
-
Find the general solution for each differential equation. dx + 3xy = 1
-
Albert owns 100% of the shares of ProTech Services Inc and is has decided to sell the business. He initially invested STG 100,000 in the shares of the business 20 years ago. He has negotiated a...
-
In a study of academic procrastination, the authors of the paper Correlates and Consequences of Behavioral Procrastination (Procrastination, Current Issues and New Directions [2000]) reported that...
-
Accounting for joint products You have just been hired as the chief cost accountant for the J&M Company. Its major product group is four food processing solutions, which are pro- duced in a joint...
-
C2.1. Changes in shareholders' equityare determined by totalearnings minusnetpayout to shareholders, butthe change in shareholders' equityis not equal to net income (in the income statement) minus...
-
The December 31, 2021, unadjusted trial balance for the Wolkstein Drug Company is presented below. December 31 is the company?s year-end reporting date. The following year-end adjusting entries are...
-
Providing for Doubtful Accounts At the end of the current year, the accounts receivable account has a debit balance of $1,043,000 and sales for the year total $11,830,000 a. The allowance account...
-
A. Richard McCarthy (born 2/14/64; Social Security number 100-10-9090) and Christine McCarthy (born 6/1/1966; Social security number 101-21-3434) have a 19-year-old son (born 10/2/99 Social Security...
-
In Exercises, the probability density function of a random variable is defined. (a) Find the expected value to the nearest hundredth. (b) Find the variance to the nearest hundredth. (c) Find the...
-
An influenza epidemic spreads at a rate proportional to the product of the number of people infected and the number not yet infected. Assume that 100 people are infected at the beginning of the...
-
Two identical flasks are each filled with a gas at 20 C and 100 atm. One flask contains NH 3 and the other an equal amount of H 2 . (a) In which flask is the collision rate with the walls of the...
-
Identify whether the following statements are true or false: a. U.S. GAAP is universally accepted in all countries in the world. b. U.S. GAAP is established by the IASB. c. Once established, U.S....
-
Determine the missing amount in each of the following cases: Assets Liabilities $190,000 $62,000 ? $53,000 $115,000 ? Stockholders' Equity ? $31,000 $61,000
-
For the following four unrelated situations, A through D, calculate the unknown amounts appearing in each column: A B C Beginning Assets. $45,000 $32,000 $53,000 ? Liabilities.. 32,000 15,000 49,000...
-
On December 31, Greg Jones completed his first year as a financial planner. The following data are available from his accounting records: a. Compute Greg's net income for the year just ended using...
-
The Benson Company has collected the following production cost data: {Required:} What would be the incremental production costs for an additional 10 units after the Benson Company has produced 8...
-
Fill in the blank. 263 kl = ________ m 3
-
Nitrogen monoxide reacts with hydrogen as follows: 2NO(g)+ H2(g) N2O(g) + H2O(g) The rate law is [H2]/ t = k[NO]2[H2], where k is 1.10 107 L2/(mol2s) at 826oC. A vessel contains NO and H2 at...
-
Find derivatives of the functions defined as follows. y = xe x
-
In Exercises 714, find f[g(x)] and g[f(x)]. f(x) = Vx + 1; g(x) = -1 X
-
Find the derivative of each function defined as follows. y 6 7 + 3 = + 5 X
-
A proposed $2.5 M investment in new equipment at a 100 MG/y M&Ms factory will save the plant $800,000/y in energy costs. Assuming an annual interest rate of 5%/y (compounded annually), and an...
-
Brief Exercise 10-7 Coronado Company obtained land by issuing 2,250 shares of its $14 par value common stock. The land was recently appraised at $103,240. The common stock is actively traded at $44...
-
The following schedule reconciles Cele Co.'s pretax GAAP income Pretax GAAP income Nondeductible expense for fines Tax deductible depreciation in excess of GAAP depreciation expens Taxable rental...

Study smarter with the SolutionInn App


