In physical chemistry, it is shown that the pressure P of a gas is related to the
Question:
In physical chemistry, it is shown that the pressure P of a gas is related to the volume V and temperature T by van der Waals’ equation:
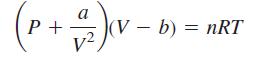
where a, b, n, and R are constants. The critical temperature Tc of the gas is the highest temperature at which the gaseous and liquid phases can exist as separate phases.
a. When T = Tc, the pressure P is given as a function P(V) of volume alone. Sketch the graph of P(V).
b. The critical volume Vc is the volume for which P'(Vc) = 0 and P"(Vc) = 0. Show that Vc = 3b.
c. Find the critical pressure Pc = P(Vc) and then express Tc in terms of a, b, n, and R.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Calculus For Business, Economics And The Social And Life Sciences
ISBN: 9780073532387
11th Brief Edition
Authors: Laurence Hoffmann, Gerald Bradley, David Sobecki, Michael Price
Question Posted:





