a. Copy the following table and complete it by writing the structural formulae for methanol and methanoic
Question:
a. Copy the following table and complete it by writing the structural formulae for methanol and methanoic acid.

b. Describe a simple chemical test that could be used to distinguish methanol from methanoic acid.
c. (i) Name the class of compound produced when methanol reacts with methanoic acid.
(ii) Name the type of reaction taking place.
(iii) Write a word and balanced chemical equation for this reaction.
(iv) Give two uses related to the class of compound formed in this reaction.
d. The following reaction takes place when methanol is burned:
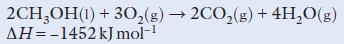
(i) How much heat energy would be liberated by burning:
0.5 mol of methanol?
4.0 mol of methanol?
4 g of methanol?
(ii) Calculate the volume of carbon dioxide produced at room temperature and pressure (rtp) when 16 g of methanol are burned.
(Ar: H = 1; C = 12; O = 16. One mole of any gas at rtp occupies 24 dm3.)
Step by Step Answer:






