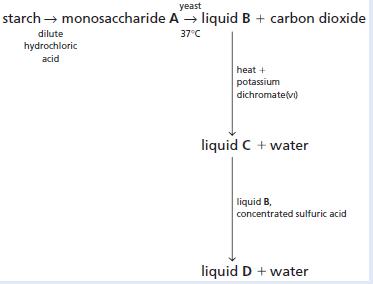
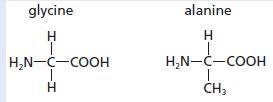
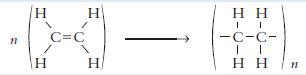
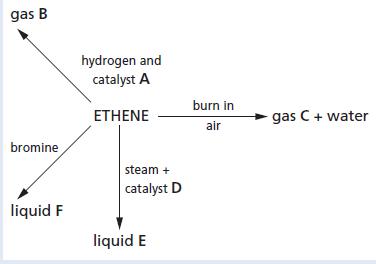
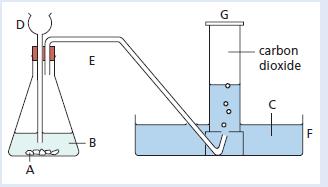
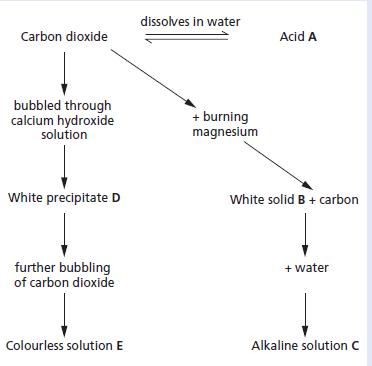
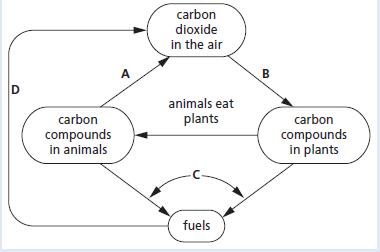
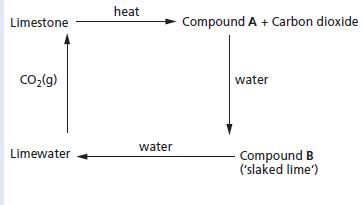
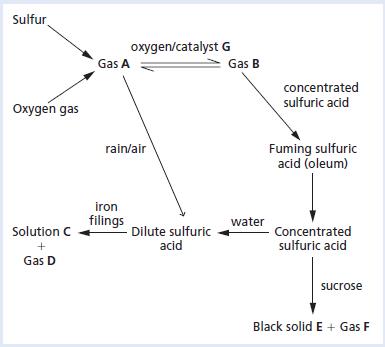
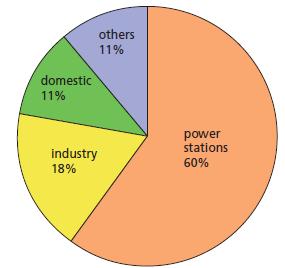
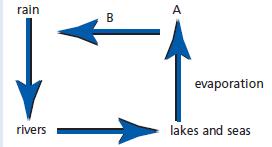
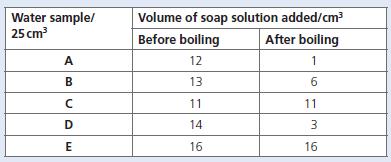
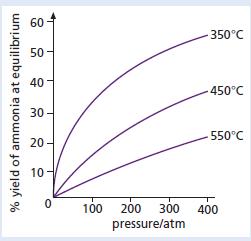
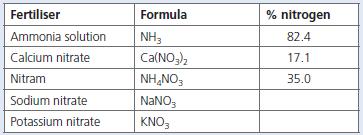
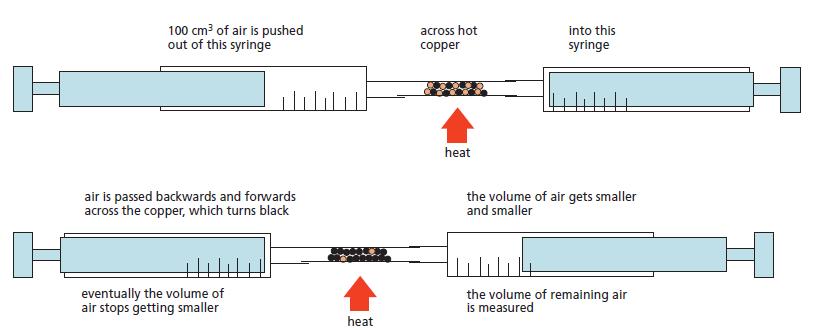
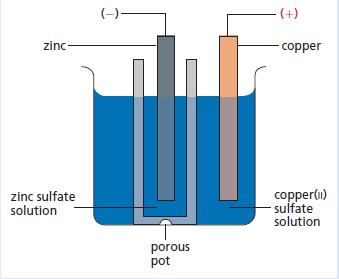
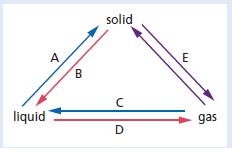
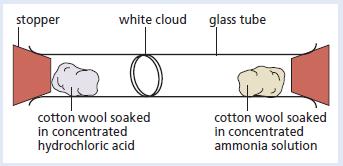
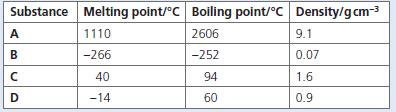
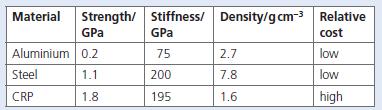
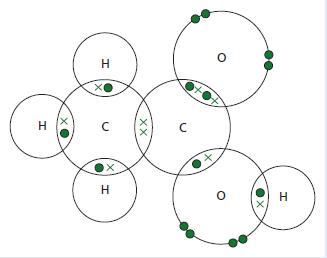
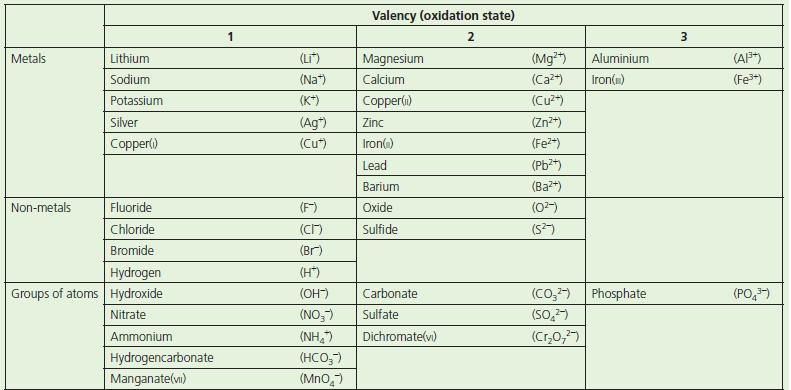
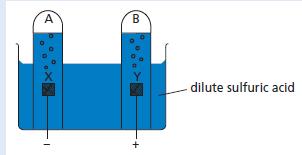
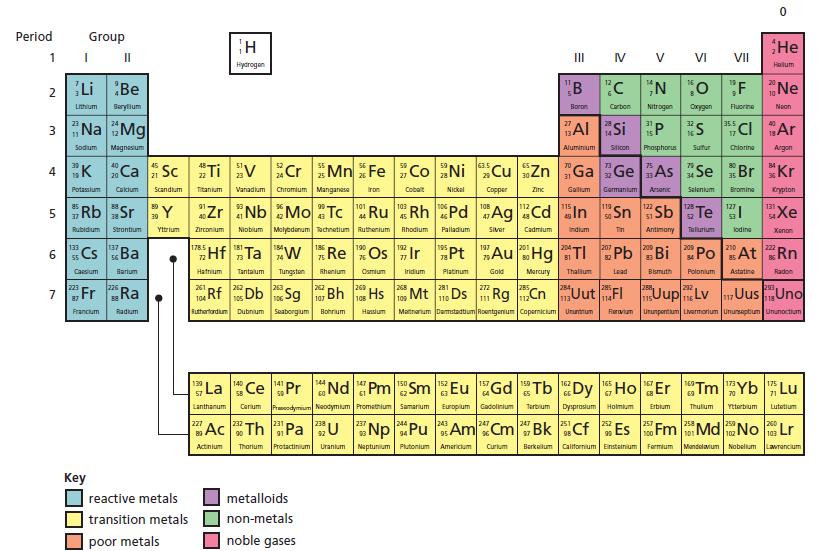
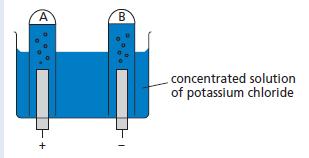
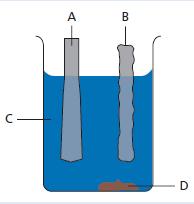
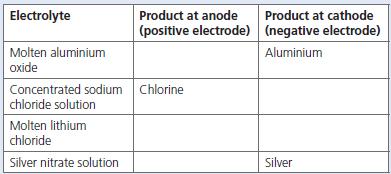
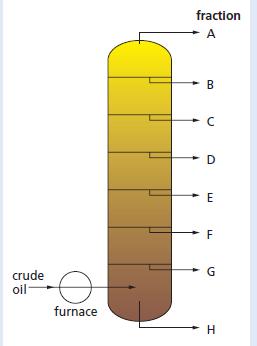
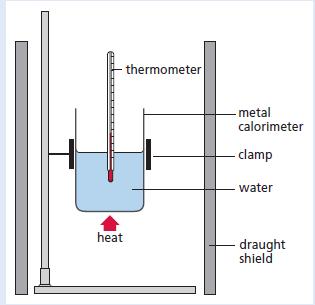
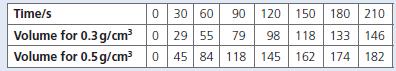
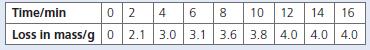
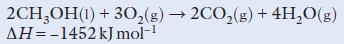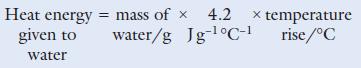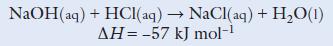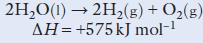Chemistry 3rd Edition Bryan Earl, Doug Wilford - Solutions
Discover comprehensive solutions to "Chemistry 3rd Edition" by Bryan Earl and Doug Wilford. Our platform offers a detailed solution manual, providing step-by-step answers to all textbook questions and solved problems. Access the answers key and chapter solutions online, ensuring a thorough understanding of each topic. Whether you're looking for a test bank or an instructor manual, our resources are designed to support your learning journey. Download solutions in PDF for free, enhancing your study experience with easy-to-follow explanations. Embrace the convenience of having all questions and answers at your fingertips.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()