Copy and complete the table below, which shows the results of the electrolysis of four substances using
Question:
Copy and complete the table below, which shows the results of the electrolysis of four substances using inert electrodes.
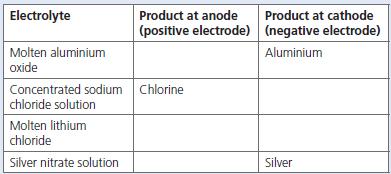
a. State what you understand by ‘inert electrodes’.
b Explain why the lithium chloride solution becomes progressively more alkaline during electrolysis.
c. Explain why solid lithium chloride is a nonconductor of electricity, whereas molten lithium chloride and lithium chloride solution are good conductors of electricity.
d. During the electrolysis of molten aluminium chloride (AlCl3) the carbon anodes are burned away. Explain why this should happen and write balanced chemical equations for the reactions that take place.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





