The apparatus shown below was set up. Give explanations for the following observations. a. The formation of
Question:
The apparatus shown below was set up.
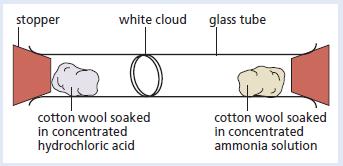
Give explanations for the following observations.
a. The formation of a white cloud.
b. It took a few minutes before the white cloud formed.
c. The white cloud formed further from the cotton wool soaked in ammonia.
d. Cooling the concentrated ammonia and hydrochloric acid before carrying out the experiment increased the time taken for the white cloud to form.
Transcribed Image Text:
stopper white cloud glass tube cotton wool soaked in concentrated hydrochloric acid cotton wool soaked in concentrated ammonia solution
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
a the Vapours from the two gases diffuse towards each other and where they meet the...View the full answer

Answered By

Amruta Hajare
I have bachelors degree in general science commonly known as BSC. After teaching vocational colleges for some years I realized my calling to teach was for young minds, giving them strong foundation for future careers and then went back to University and did post graduate diploma in education (PGDE). I am therefore experienced tutor tackling various topics in Biology, chemistry and mathematics under IGCSE curriculum. I give services to my clients at a fair price and relate very well with both parents and students.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Concentrated hydrochloric acid contains 1.00 mol HCl dissolved in 3.31 mol H2O. What is the mole fraction of HCl in concentrated hydrochloric acid? What is the molal concentration of HCl?
-
A barium mineral was dissolved in hydrochloric acid to give a solution of barium ion. An excess of potassium sulfate was added to 50.0 mL of the solution, and 1.128 g of barium sulfate precipitate...
-
Concentrated hydrochloric acid is usually available at a concentration of 37.7 percent by mass. What is its molar concentration? (The density of the solution is 1.19 g/mL.)
-
In Exercises find the derivative of the function. y = x(x + 1)
-
A weak acid, HA, is dissolved in water. Which one of the following beakers represents the resulting solution? (Water molecules have been omitted for clarity.)
-
This exercise continues the Lawlor Lawn Service, Inc., situation from Exercise 4-36 of Chapter 4. Lawlor Lawn Service has also begun selling plants that it purchases from a wholesaler. During June,...
-
[!J. This exercise is used in Section ell. 7. Let F : R3 --+ R be continuously differentiable at (a, b, c) with 'V F (a, b, c) :f:. O. (a) Prove that the graph of the relation F(x, y, z) = 0; Le.,...
-
Dirkson Company and Hawkins Corporation, two corporations of roughly the same size, are both involved in the manufacture of in-line skates. Each company depreciates its plant assets using the...
-
Please explain how to calculate this to get an answer like the key above (check the picture for the complete question) for number 50, 51, 52, 53, 54, 55, 56, 57, and 58 Chapter : INVESTMENTS 50....
-
A storeroom is used to organize items stored in it on N shelves. Shelves are numbered from 0 to N-1. The K-th shelf is dedicated to items of only one type, denoted by a positive integer A[K]....
-
Define the following terms using specific examples to help with your explanation: a. Element b. Metal c. Non-metal d. Compound e. Molecule f. Mixture g. Flocculation h. Gel i. Foam j. Emulsion k. Sol.
-
Use the kinetic theory to explain the following: a. When you take a block of butter out of the fridge, it is quite hard. However, after 15 minutes it is soft enough to spread. b. When you come home...
-
Use the data in NutritionStudy to assess the conditions for doing inference on a regression model to predict a persons cholesterol level, Cholesterol, from the daily grams of fat, Fat. Explain your...
-
Country Analysis of Milan: -Discuss the overall cultural, political, legal, economic, and technological infrastructure issues that could potentially impact a product's introduction in Milan. -You...
-
Sarah is a highly introverted employee who works in a fast-paced sales team. Her manager recently noticed that Sarah tends to be reserved during team meetings and rarely volunteers her ideas....
-
Community Disaster Preparedness Review the information found at https://www.ready.gov/community-preparedness-toolkit Look at the steps involved in formulating community response to a disaster....
-
The topic of diversity, tolerance, and inclusion has been widely (and, in many cases, hotly) debated in society over the past several years. In my opinion (and this is up for discussion - hence this...
-
The objective of this coursework is for you to critically engage with the theory and practice of promoting wellbeing or equality, diversity and inclusion (including cultural diversity) for employees...
-
The financial statements of M&S are presented in Appendix E. The companys complete annual report, including the notes to the financial statements, is available online. Instructions Refer to M&Ss...
-
Find the equation of the plane passing through the points P 5,4,3 ,Q 4,3,1 and R 1,5,4
-
Prove that the perfect gas temperature scale and the thermodynamic temperature scale based on the Second Law of thermodynamics differ from each other by at most a constant numerical factor.
-
Evaluate (ClS/ClV)]' for (a) A van der Waals gas, (b) A Dieterici gas (Table 1.7). For an isothermal expansion, for which kind of gas (and a perfect gas) will /).5be greatest? Explain your conclusion.
-
Two of the four Maxwell relations were derived in the text, but two were not. Complete their derivation by showing that (S/V)T = (p/T)V (T/P)s = (V/S)p
-
ACC 2 0 2 Milestone One: Operational Costs Data Appendix You plan to open a small business for manufacturing pet collars, leashes, and harnesses. You have found a workshop space you can use for...
-
Explain the following: Understand the PPE acquisition (or investing) cycle and related significant transactions and source documents Understand the relevant assertions/objectives about PPE balances...
-
Problem 3 Progress Company acquired 6 0 % of Stall Corporation on 1 2 0 2 0 . Fair values of Stall's assets and liabilities approximated book values on that date. Progress uses the initial value...

Study smarter with the SolutionInn App


