Carbon-fibre-reinforced plastic (CRP) is used in the manufacture of golf clubs and tennis rackets. a. What are
Question:
Carbon-fibre-reinforced plastic (CRP) is used in the manufacture of golf clubs and tennis rackets.
a. What are composite materials?
b. Which two substances are used to manufacture this composite material? Consider the data in the table.
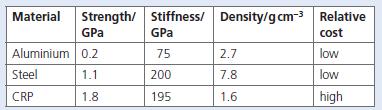
c. Discuss the advantages and disadvantages of using the three materials above in the manufacture of golf clubs.
Transcribed Image Text:
Stiffness/ Density/gcm-3 Relative GPa Material Strength/ GPa cost Aluminium 0.2 75 2.7 low Steel 1.1 200 7.8 low CRP 1.8 195 1.6 high
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
a Com pos ite materials are materials composed of two or more different substances The different sub...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The head box process is used in the manufacture of paper to transform the pulp slurry flow into a jet of 2 cm and then spread it onto a mesh belt [22]. To achieve desirable paper quality, the pulp...
-
A press produces parts used in the manufacture of large-screen plasma televisions. If the press is correctly adjusted, it produces parts with a scrap rate of 5%. If it is not adjusted correctly, it...
-
Carbon tetrachloride is a colorless liquid used in the manufacture of fluorocarbons and as an industrial solvent. How many molecules are there in 7.58 mg of carbon tetrachloride?
-
Redo Exercise 29 for the situation in which Ms. Jones withdrew $1000 at the end of the seventh year instead of depositing it. Data in Exercise 29 Ms. Jones deposited $100 at the end of each month for...
-
Give the conjugate acid to each of the following species regarded as bases. a. HSe b. NH2 c. ClO2 d. N2H4
-
One year ago, Tyler Stasney founded Swift Classified Ads. Stasney remembers that you took an accounting course while in college and comes to you for advice. He wishes to know how much net income his...
-
6. Let V be an open set in Rn, a E V, and f : V ---+ R be C2 on V. If f (a) is a local minimum of f, prove that D(2) f (a) (h) 2: 0 for all h ERn.
-
Innovative Office Inc. has cash and carry customers and credit customers. Innovative Office estimates that 30% of monthly sales are to cash customers, while the remaining sales are to credit...
-
What is the formula for the present value of $50,000, to be received 4 years from now, with a 10% per year discount rate compounded semi-annually? $50,000 = (1.05)8 $50,000 + (1.1094 $50,000 X (1.10)...
-
a. Formulate and solve a binary integer programming problem to maximize the total number of kitchen sets (and thus the number of customer orders) Furniture City stocks in the local warehouse. Assume...
-
a. Define the terms: proton, neutron and electron. b. An atom X has a proton number of 19 and relative atomic mass of 39. (i) How many electrons, protons and neutrons are there in an atom of X? (ii)...
-
a. How many atoms of the different elements are there in the formulae of the compounds given below? (i) Nitric acid, HNO 3 (ii) Methane, CH 4 (iii) Copper nitrate, Cu(NO 3 ) 2 (iv) Ethanoic acid, CH...
-
For each of the following events, identify whether it is an external event that would be recorded as a transaction (E), an internal event that would be recorded as a transaction (I), or not recorded...
-
Companies that invest heavily in eco-friendly initiatives, such as transitioning to renewable energy sources or implementing carbon offset programs, may initially face increased operational costs....
-
Answer each question individually please. 14-13 What are the advantages and drawbacks of universities using social media to communicate with various stakeholdersstudents, potential students, alumni,...
-
act as a consultant hired by the operations director of the Barry Computer Company provide a financial analysis and comparison to the industry. You will conduct a financial ratio analysis to gain a...
-
Building a sense of community is not just a moral thing to do, but also a pragmatic one. In today's competitive and ever-evolving business environment, the organizations that can attract the most...
-
Watch https://youtu.be/U3MtvvNjUR4 What do you think of Dr. Saint's ideas about barriers to change? What do you think about social learning? Could this tool be used to make real change? How can the...
-
Scorcese Inc. is involved in a lawsuit at December 31, 2020. (a) Prepare the December 31 entry assuming it is probable that Scorcese will be liable for $900,000 as a result of this suit. (b) Prepare...
-
A police officer pulls you over and asks to search your vehicle because he suspects you have illegal drugs inside your car. Since he doesn't have reasonable suspicion to search your car, legally he...
-
Charles's law is sometimes expressed in the form V = Vo (l + a), where is the Celsius temperature, a is a constant, and Vo is the volume of the sample at Oe. The following values for a have been...
-
A constant-volume perfect gas thermometer indicates a pressure of 6.69 kPa at the triple point temperature of water (273.16 K). (a) What change of pressure indicates a change of 1.00 K at this...
-
Calculate the molar volume of chlorine gas at 350 K and 2.30 atm using (a) The perfect gas law and (b) The van der Waals equation. Use the answer to (a) to calculate a first approximation to the...
-
question 6 Timely Inc. produces luxury bags. The budgeted sales and production for the next three months are as follows july. august september Sales, in units 1,115. 1229. 1302 Production. in units...
-
On May 12 Zimmer Corporation placed in service equipment (seven-year property) with a basis of $220,000. This was Zimmer's only asset acquired during the year. Calculate the maximum depreciation...
-
Power Manufacturing has equipment that it purchased 7 years ago for $2,550,000. The equipment was used for a project that was intended to last for 9 years and was being depreciated over the life of...

Study smarter with the SolutionInn App


