a. Ethene, C 2 H 4 , is the starting material for making plastic carrier bags. (i)
Question:
a. Ethene, C2H4, is the starting material for making plastic carrier bags.
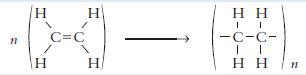
(i) Name the type of chemical change taking place in the diagram above.
(ii) Name the product formed by this reaction.
(iii) The alkene, ethene, is made by cracking large alkane molecules. Describe a simple chemical test to show that ethene is present.
b. The majority of carrier bags are difficult to dispose of.
(i) Explain why carrier bags should not just be thrown away.
(ii) Explain why the majority of plastic carrier bags are recycled.
(iii) Give one advantage that a plastic carrier bag has over one made out of paper.
c. A label like the one below is found on some plastic carrier bags.
(i) What is the meaning of the term element?
(ii) What is the name given to the type of compound that contains the elements carbon and hydrogen only?
(iii) When the plastic bag burns, heat energy is given out. What name is used to describe reactions that give out heat energy?
(iv) The plastic bag will probably give off a toxic gas when it is burned. Why is this the case?
This plastic carrier bag is made from a substance that is made from the chemical elements carbon and hydrogen only. When the carrier bag is burned it produces carbon dioxide and water. These substances are natural and will not harm the environment.
Step by Step Answer:






