One of the first practical chemical cells was the Daniell cell invented by John Daniell in 1836.
Question:
One of the first practical chemical cells was the Daniell cell invented by John Daniell in 1836. A diagram of this type of cell is shown below.
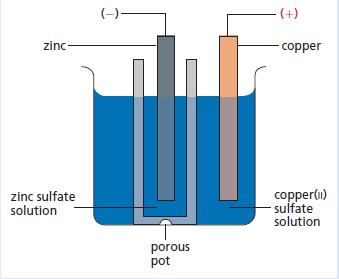
It is capable of generating about 1.1 volts and was used to operate small electrical items such as doorbells.
a. The electrode reaction taking place at a copper anode is:
Cu2+ (aq) + 2e− → Cu(s)
Write an electrode equation for the process taking place at the cathode.
b. Which way would the electrons flow in the wire connected to the voltmeter – from ‘copper to zinc’ or ‘zinc to copper’?
c. Why should copper(ii) sulfate crystallise at the bottom of the outer container?
d. What is the function of the porous pot?
e. There are problems associated with the Daniell cell which have led to it being replaced by other types of cell. Give two reasons why Daniell cells are no longer in use today.
Step by Step Answer:






