Crude oil is a mixture of hydrocarbons. The refining of crude oil produces fractions which are more
Question:
Crude oil is a mixture of hydrocarbons. The refining of crude oil produces fractions which are more useful to us than crude oil itself. Each fraction is composed of hydrocarbons which have boiling points within a specific range of temperature. The separation is carried out in a fractionating column, as shown below.
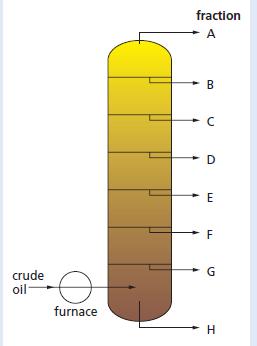
a. Which separation technique is used to separate the fractions?
b. Name each of the fractions A to H and give a use for each.
c. Why do the fractions come from the fractionating column in this order?
d. What is the connection between your answer to c and the size of the molecules in each fraction?
e. Which of the fractions will be the most flammable?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





