The following results were obtained from an experiment carried out to measure the enthalpy of combustion (heat
Question:
The following results were obtained from an experiment carried out to measure the enthalpy of combustion (heat of combustion) of ethanol. The experiment involved heating a known volume of water with the flame from an ethanol burner.
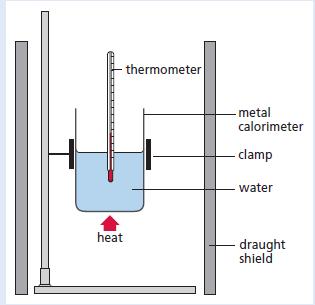
The burner was weighed initially and after the desired temperature rise had been obtained.
Volume of water in glass beaker = 200 cm3
Mass of ethanol burner at start = 85.3 g
Mass of ethanol burner at end = 84.8 g
Temperature rise of water = 12 °C
(Density of water = 1 g cm−3)
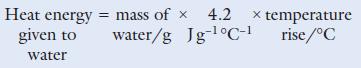
a. Calculate the mass of ethanol burned.
b. Calculate the amount of heat produced, in joules, in this experiment by the ethanol burning.
c. Convert your answer to b into kilojoules.
d. Calculate the amount of heat produced by 1 g of ethanol burning.
e. What is the mass of 1 mole of ethanol (C2H5OH)?
(Ar: H = 1; C = 12; O = 16)
f. How much heat would be produced if 1 mole of ethanol had been burned? (This is the heat of combustion of ethanol.)
g. Compare your value with the actual value of 1371 kJ mol−1 and suggest two reasons for the difference in values.
h. Write a balanced chemical equation to represent the combustion of ethanol.
Step by Step Answer:






