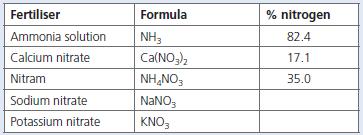
a. Copy and complete the above table by calculating the percentage of nitrogen in the fertilisers sodium
Question:
a. Copy and complete the above table by calculating the percentage of nitrogen in the fertilisers sodium nitrate and potassium nitrate.
(Ar: H = 1; N = 14; O = 16; Na = 23; K = 39; Ca = 40)
b. Including the data you have just calculated, which of the fertilisers contains:
(i) The largest percentage of nitrogen?
(ii) The smallest percentage of nitrogen?
c. Give the chemical name for the fertiliser that goes by the name Nitram®.
d. Ammonia can be used directly as a fertiliser but not very commonly. Think of two reasons why ammonia is not often used directly as a fertiliser.
e. Nitram® fertiliser is manufactured by the reaction of nitric acid with ammonia solution according to the equation:
NH3(aq) + HNO3(aq) → NH4NO3(aq)
A bag of Nitram® may contain 50 kg of ammonium nitrate. What mass of nitric acid would be required to make it?
Step by Step Answer:






