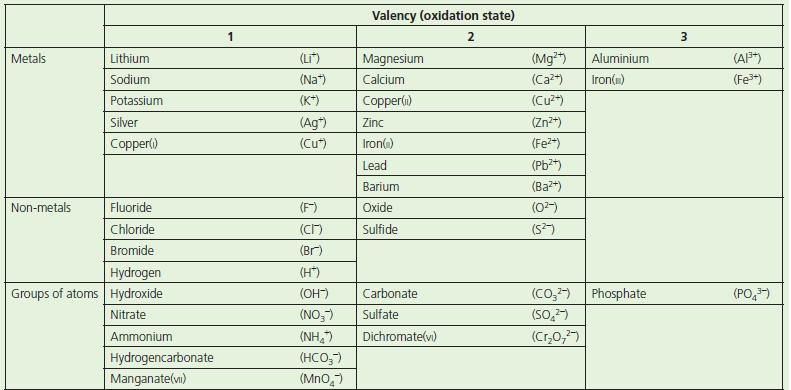
Use the information given in Table 3.6 on p. 43 to work out the formula for: a.
Question:
Use the information given in Table 3.6 on p. 43 to work out the formula for:
a. Silver oxide
b. Zinc chloride
c. Potassium sulfate
d. Calcium nitrate
e. Iron(ii) nitrate
f. Copper(ii) carbonate
g. Iron(iii) hydroxide
h. Aluminium fluoride.
Table 3.6
Valency (oxidation state) 1 Metals Lithium (LI") Magnesium (Mg) Aluminium (AP) Sodium (Na*) Calcium (Ca2") Iron(m) (Fe*) Potassium (K*) Copperi) (Cu2") Silver (Ag") Zinc (Zn") Copperl) (Cu*) Iron() (Fe2*) Lead (Pb) Barium (Ba") Non-metals Fluoride (F) Oxide (02-) Chloride (Cr) Sulfide (S2-) Bromide (Br) Hydrogen (H") Groups of atoms Hydroxide (OH") Carbonate (CO,) Phosphate (PO,) Nitrate (NO,) Sulfate (SO,) Ammonium (NH,") Dichromate(vi) (Cr,0,) Hydrogencarbonate (HCO;) Manganate(vi) (Mno,)
Step by Step Answer:
Compound name a Silver oxide b Zinc Chloride ...View the full answer



Related Video
The experiment aims to show the impact of various beverages on teeth by using eggs as a representation of enamel. Three eggs are boiled and then placed in glasses filled with fizzy drinks, vinegar, and mango juice for 24 hours. The shells of eggs are similar to enamel as they are composed of calcium carbonate, and enamel is primarily made of calcium phosphate. The eggs are then observed to demonstrate the effects of the different liquids on teeth and the importance of brushing regularly. The egg placed in fizzy drink has turned dark in color but can be cleaned by brushing with toothpaste and rinsing with water. The egg placed in vinegar has had its shell softened due to the chemical reaction of vinegar and calcium carbonate, which can\'t be reversed. This highlights the fact that acids are more damaging to teeth than other substances. The egg placed in mango juice represents the process of bacteria in the mouth converting sugars and starches into acids that form plaque, which can be prevented by brushing. The use of fluoride in toothpaste is also highlighted as it slows down the demineralization process and protects the enamel. The importance of brushing teeth twice a day is emphasized.
Students also viewed these Sciences questions
-
Use the information given in the previous exercise to determine if there is sufficient evidence to conclude that the mean pulse rate in a dental setting differs from the mean pulse rate in a medical...
-
Use the information given in Problem 2 of Chapter 8, as well as the computer output given here, to answer the following questions about the data from that problem. a. Conduct overall regression F...
-
Use the information given in the following figure to construct a mathematical model for the number of pounds of salt x 1 (t), x 2 (t), and x 3 (t) at time t in tanks A, B, and C, respectively....
-
First Ownership orders merchandise from several suppliers from around the world. Each of the suppliers has different shipping or transportation terms. At the end of December, First Ownership had the...
-
Complete each of the following equations. Then write the Lewis formulas of the reactants and products and identify each reactant as a Lewis acid or a Lewis base. a. AlCl3 + Cl b. I + I2
-
Rolling Hill Interiors, Inc., began the year with Retained earnings of $20,000. On July 12, Rolling Hill issued common stock and received $14,000 cash. The income statement for the year ended...
-
3. Evaluate the following limits using results from this section. You may assume that sinx, 1 - cos x, and ?Ix converge to 0 as x -+ O.J (a) (b) (c) (d) (e) 1. X2 + cos x 1m . x-o 2 - tan x 1. x2 + X...
-
Selected accounts of Welch Company are shown on the shown below. Instructions After analyzing the accounts, journalize (a) The July transactions (b) The adjusting entries that were made on July 31....
-
Multiple Choice Question 103 On April 15 of the current year, a fire destroyed the entire uninsured inventory of a retail store. The following data are available: Sales, January 1 through April 15...
-
Why might a big company like Lenovo want to develop strategic partnerships with locally owned computer stores? Describe what Lenovo would have to do to maintain such relationships.
-
Atoms of elements X, Y and Z have 16, 17 and 19 electrons, respectively. Atoms of argon have 18 electrons. a. Determine the formulae of the compounds formed by the combination of the atoms of the...
-
The diagram shows the arrangement of the outer electrons only in a molecule of ethanoic acid. a. Name the different elements found in this compound. b. What is the total number of atoms present in...
-
Contrary to what you might expect, a solid steel ball can float on water due to the surface tension effect. Determine the maximum diameter of a steel ball that would float on water at 20C. What would...
-
In addition to the strongest military in the world, the United States wields enormous soft power. Define soft power. What factors make the United States powerful when it comes to soft power?
-
Tampa by the Bay Cardiology practice is experiencing long wait times for new patient appointments. Next available appointment is 30 days. The administrator has asked the practice manager to construct...
-
In 2013, Idalia Hernndez Ramos, a middle school teacher in Mexico, was a victim of cyber harassment. After discovering that one of her students tweeted that the teacher was a "bitch" and a "whore,"...
-
Your life couldn't be any better. You just accepted a new role as a senior consultant for a project management services firm in San Francisco, and the move is finally happening. You've got a great...
-
What are the two "engines" that drive earth's processes, how do they work (basically) and what are their energy sources? How do the "engines" influence and interact with the Earth Systems? (provide a...
-
Sycamore Candy offers an MP3 download (seven-single medley) as a premium for every five candy bar wrappers presented by customers together with $2.50. The candy bars are sold by the company to...
-
Repeat Exercise 16.6 using the t-test of the coefficient of correlation. Is this result identical to the one you produced in Exercise 16.6?
-
A gas mixture consists of 320 mg of methane, 175 mg of argon, and 225 mg of neon. The partial pressure of neon at 300 K is 8.87 kPa. Calculate (a) The volume and (b) The total pressure of the mixture.
-
In an experiment to measure the molar mass of a gas, 250 cm3 of the gas was confined in a glass vessel. The pressure was 152 Torr at 298 K and, after correcting for buoyancy effects, the mass of the...
-
A certain sample of a gas has a volume of20.00 dm ' at OCand 1.000 atm. A plot of the experimental data of its volume against the Celsius temperature, , at constant p, gives a straight line of slope...
-
A company is evaluating a new 4-year project. The equipment necessary for the project will cost $3,300,000 and can be sold for $650,000 at the end of the project. The asset is in the 5-year MACRS...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
I need to see where the calculations for this problem come from plz. 5. Award: 4.00 points Lucido Products markets two computer games: Claimjumper and Makeover. A contribution format income statement...

Study smarter with the SolutionInn App


