a. Name and give the formulae of substances A to E. b. Write balanced chemical equations for
Question:
a. Name and give the formulae of substances A to E.
b. Write balanced chemical equations for the reactions in which compounds B, C and E were formed.
c. Where would you expect to find acid A?
d. Universal indicator solution was added to solution C. What colour did it go?
e. Upon addition of dilute hydrochloric acid to solution C, a neutralisation reaction took place.
(i) Write a balanced chemical equation for the reaction taking place.
(ii) Name the salt produced in this reaction.
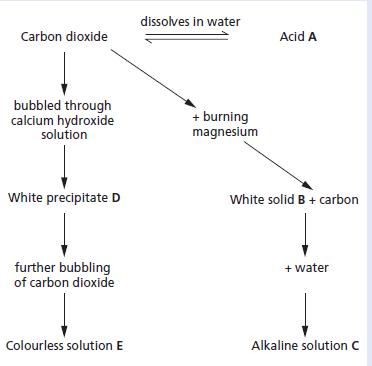
dissolves in water Carbon dioxide Acid A bubbled through calcium hydroxide solution + burning magnesium White precipitate D White solid B + carbon further bubbling of carbon dioxide + water Colourless solution E Alkaline solution C
Step by Step Answer:
a b A HCO3 C MgOH2 E CaHCO32 for B CO g Mg s for C ...View the full answer



Related Video
The experiment aims to show the impact of various beverages on teeth by using eggs as a representation of enamel. Three eggs are boiled and then placed in glasses filled with fizzy drinks, vinegar, and mango juice for 24 hours. The shells of eggs are similar to enamel as they are composed of calcium carbonate, and enamel is primarily made of calcium phosphate. The eggs are then observed to demonstrate the effects of the different liquids on teeth and the importance of brushing regularly. The egg placed in fizzy drink has turned dark in color but can be cleaned by brushing with toothpaste and rinsing with water. The egg placed in vinegar has had its shell softened due to the chemical reaction of vinegar and calcium carbonate, which can\'t be reversed. This highlights the fact that acids are more damaging to teeth than other substances. The egg placed in mango juice represents the process of bacteria in the mouth converting sugars and starches into acids that form plaque, which can be prevented by brushing. The use of fluoride in toothpaste is also highlighted as it slows down the demineralization process and protects the enamel. The importance of brushing teeth twice a day is emphasized.
Students also viewed these Sciences questions
-
In what chemical shift ranges would you expect to find the proton NMR signals of ethyl acetate (CH3CO2CH2CH3)?
-
What kind of organizational structure would you expect to find in (a) A fast-food restaurant (b) A company like GE or GM (c) A biotechnology company?
-
Would you expect to find adenineguanine or cytosinethymine base pairs in DNA? Why?
-
Factor each polynomial. 64y 9 + z 6
-
A solution was prepared by dissolving 0.834 g of sulfur, S8, in 100.0 g of acetic acid, HC2H3O2. Calculate the freezing point and boiling point of the solution.
-
Discuss the following statement: Health care costs are out of control in the United States, and increasing conflicts between employers and employees are likely as employers try to reduce their health...
-
What type of information about stakeholders is not included in a stakeholder register? a. identification b. classification c. assessment d. engagement level LO.1
-
What criteria must be met before donated services can be recorded as contribution revenue and an expense? Give an example of a service that might qualify as a donated service for accounting purposes.
-
Comprehensive Problem 5-2B John Fuji (birthdate June 6, 1981) received the following Form W-2 from his employer related to his job as a manager at a Washington apple-processing plant are reported on...
-
Elm Manufacturing Company (ELM) is a small manufacturer of back packs located in Rochelle, Illinois. They make three different types of Backpacks: A small backpack made for school that is designed to...
-
Limestone is an important raw material used in many different industries. a. One of the properties of limestone is that it reacts with acids. (i) Why do farmers spread powdered limestone on their...
-
The following question is about carbon dioxide. a. Name and give the formula of each of the substances A, B and C. b. Identify by name the different pieces of apparatus D, E, F and G. c. Draw and...
-
The auditor, in recommending controls, should always consider the cost of the control in relation to the risk. Which of the following controls best reflects this philosophy with respect to a large...
-
2. If w = x + y z + sint and x + y = t, find Iw dw Iw a. b. c. av z x, z aw Iw aw d. e. f. az at at y, t x, Z y, z
-
Global Training Solutions (GTS) was founded 40 years ago by an educator/consultant/trainer to provide training to business employees, consulting services to businesses and school systems, and...
-
Consider the following open-loop transfer function: K(s+2a) G(s)H(s)= where K,a>0 s(s-a) The frequency when the phase is -180 degrees is: rad/s The magnitude of the open loop transfer function when...
-
Complete Exhibit 5 (this should be cost per cup and in CA$, do not convert). The exhibit is missing a line for Milk & Sugar within factory overhead section EXHIBIT 5: COSTING CHART Direct Cost Coffee...
-
Why is the tension negative in the net force x-component? The tension points right, so shouldn't it be positive? I saw elsewhere that the equation has negative T. Why? Chrome File Edit View History...
-
Brown Industries has a debt-equity ratio of 1.5. Its WACC is 9.6 percent, and its cost of debt is 5.7 percent. There is no corporate tax. a. What is the companys cost of equity capital? b. What would...
-
Use the graphs of f and g to graph h(x) = (f + g) (x). To print an enlarged copy of the graph, go to MathGraphs.com. 1. 2. y 24 8. 2. -2 -2 4 6
-
A photon-powered spacecraft of mass 10.0 kg emits radiation of wavelength 225 nm with a power of 1.50 kW entirely in the backward direction. To what speed will it have accelerated after 10.0 y if...
-
A laser used to read CDs emits red light of wavelength 700 nm. How many photons does it emit each second if its power is? (a) 0.10 W, (b) LOW?
-
The work function for metallic cesium is 2.14 eV. Calculate the kinetic energy and the speed of the electrons ejected by light of wavelength (a) 700 nm, (b) 300 nm.
-
A government bond matures in 30 years, makes semi-annual coupon payments of 6.0% ($120 per year) and offers a yield of 3.7% annually compounded. Assume face value is $1,000. Three years later the...
-
Your objective is: 1. Carry out a life insurance needs analysis, for each one of them (show your calculations) [30 Marks] 2. Refer to the case and the insurance plan quotes. Would you recommend...
-
TufStuff, Incorporated, sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the company s products is a heavy - duty corrosion - resistant...

Study smarter with the SolutionInn App


