A flask containing dilute hydrochloric acid was placed on a digital balance. An excess of limestone chippings
Question:
A flask containing dilute hydrochloric acid was placed on a digital balance. An excess of limestone chippings was added to this acid, a plug of cotton wool was placed in the neck of the flask and the initial mass was recorded. The mass of the apparatus was recorded every two minutes. At the end of the experiment the loss in mass of the apparatus was calculated and the following results were obtained.
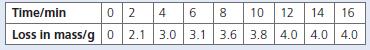
a. Plot the results of the experiment.
b. Which of the results would appear to be incorrect? Explain your answer.
c. Write a balanced chemical equation to represent the reaction taking place.
d. Why did the mass of the flask and its contents decrease?
e. Why was the plug of cotton wool used?
f. How does the rate of reaction change during this reaction? Explain this using particle theory.
g. How long did the reaction last?
h. How long did it take for half of the reaction to occur?
Step by Step Answer:






