A student performed two experiments to establish how effective manganese(iv) oxide was as a catalyst for the
Question:
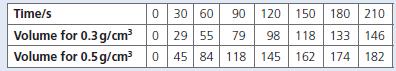
A student performed two experiments to establish how effective manganese(iv) oxide was as a catalyst for the decomposition of hydrogen peroxide. The results below were obtained by carrying out these experiments with two different quantities of manganese(iv) oxide. The volume of the gas produced was recorded against time.
a. Draw a diagram of the apparatus you could use to carry out these experiments.
b. Plot a graph of the results.
c. Is the manganese(iv) oxide acting as a catalyst in this reaction? Explain your answer.
d. (i) At which stage does the reaction proceed most quickly?
(ii) How can you tell this from your graph?
(iii) In terms of particles, explain why the reaction is quickest at the point you have chosen in (i).
e. Why does the slope of the graph become less steep as the reaction proceeds?
f. What volume of gas has been produced when using 0.3 g of manganese(iv) oxide after 50 s?
g. How long did it take for 60 cm3 of gas to be produced when the experiment was carried out using 0.5 g of the manganese(iv) oxide?
h. Write a balanced chemical equation for the decomposition of hydrogen peroxide.
Step by Step Answer:





