The table below shows the melting points, boiling points and densities of substances A to D. a.
Question:
The table below shows the melting points, boiling points and densities of substances A to D.
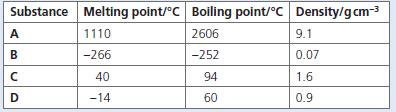
a. Which substance is a gas at room temperature?
b. Which substance is a liquid at room temperature?
c. Which substances are solids at room temperature?
d. Which substance is most likely to be a metal?
e. Which substance will be a liquid at −260 °C?
f. What is the melting point of the least dense nonmetal?
g. Which substances are gases at 72 °C?
Transcribed Image Text:
Substance Melting point/°C Boiling point/°C Density/gcm-3 A 1110 2606 9.1 -266 -252 0.07 40 94 1.6 D -14 60 0.9
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
Answers According to The International Union of Pure and Applied Chemistry IUPAC the standard room ...View the full answer

Answered By

G Sampath
A Global Online Tutor having 9+ years of experience with a Master’s Degree in Organic Chemistry. Have been tutoring chemistry to the students of different country curriculum's according to their subject needs. Have made many students fall in love with chemistry and delivered high-quality, result-oriented, personalized, one-on-one live video/audio/chat enabled sessions. I have strong theoretical and practical knowledge in all areas of chemistry and the ability to teach the students in an easy and understandable manner.I assure you of providing the highest quality work on any assigned task before the mentioned time.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
The table below shows the stock price, earnings per share, and dividends per share for three companies as of October 2007: (a) Determine the price-earnings ratio and dividend yield for the three...
-
The table below shows the stock price, earnings per share, and dividends per share for three companies as of October 2007: a. Determine the price-earnings ratio and dividend yield for the three...
-
The table below shows the closing monthly stock prices for Amazon.com and Google during 2007. Calculate the simple three-month moving average for each month for bothcompanies. 56810 41397 57 19817...
-
Hanley asks his assistant to collect details on those costs included in the $21,000 indirect-cost pool that can be traced to each individual job. After analysis, Wigan is able to reclassify $14,000...
-
Give the conjugate base to each of the following species regarded as acids. a. HSeO4 b. PH4+ c. HS d. HOCl
-
Grant Film Productions wishes to expand and has borrowed $100,000. As a condition for making this loan, the bank requires that the business maintain a current ratio of at least 1.50. Business has...
-
4. Let f : Rn ---+ Rm be differentiable at a, and 9 : Rm ---+ R be differentiable at b = f(a). Prove that if g (b) is a local extremum of g, then '\1(g 0 f) (a) = O.
-
A gas-turbine power plant operates on the simple Brayton cycle between the pressure limits of 100 and 700 kPa. Air enters the compressor at 30C at a rate of 12.6 kg/s and leaves at 260C. A diesel...
-
Elegance is a manufacturer of large flower pots for urban settings. The company has these standards: (Click the icon to view the standards.) (Click the icon to view the actual results.) Prepare a...
-
The TitMar Motor Company is considering the production of a new personal transportation vehicle (PTV). The PTV would compete directly with the innovative new Segway. The PTV will utilize a...
-
a. How many atoms of the different elements are there in the formulae of the compounds given below? (i) Nitric acid, HNO 3 (ii) Methane, CH 4 (iii) Copper nitrate, Cu(NO 3 ) 2 (iv) Ethanoic acid, CH...
-
Name the method which is most suitable for separating the following: a. Oxygen from liquid air b. Red blood cells from plasma c. Petrol and kerosene from crude oil d. Coffee grains from coffee...
-
Reverse the slope of the line in Exercise 12.51 by reordering the y observations, as follows: Repeat the steps of Exercise 12.51. Notice the change in the sign of r and the relationship between the...
-
Create your own privacy philosophy. This should cover the policies you will use for email, texting, social media, and internet usage. Consider what information is being collected anout you in each...
-
1. What future markets might be attractive to Carrefour and which mode of operation would be preferable? How important is the theoretical concept of psychological distance? 2. Corporate...
-
Briefly restate your problem space and methodology. Considering your problem space and methodology, what factors are you considering in deciding whether to use a theoretical foundation or a...
-
In light of your personal experience, what strategies or approaches do you believe could be effective in creating a workplace environment where employees from diverse cultural backgrounds feel both...
-
1. Entrepreneurs hold many common traits, identify five common traits of an entrepreneur that resonate with you and discuss each one of the five traits and why they matter to you. 2. Why is...
-
Soundgarden Company sold 200 color laser copiers on July 10, 2020, for $4,000 apiece, together with a 1-year warranty. Maintenance on each copier during the warranty period is estimated to be $330....
-
we have to compute the letter grades for a course. The data is a collection of student records stored in a file. Each record consists of a name(up to 20 characters), ID (8 characters), the scores of...
-
Express the van der Waals equation of state as a virial expansion in powers of 1/Vm and obtain expressions for Band C in terms of the parameters a and b. The expansion you will need is (1- xtI = 1 +...
-
The second virial coefficient B' can be obtained from measurements of the density p of a gas at a series of pressures. Show that the graph of p/ p against p should be a straight line with slope...
-
The following equations of state are occasionally used for approximate calculations on gases: (gas A) p Vm = RT(1 + b/V m)' (gas B) p(V m - b) = RT. Assuming that there were gases that actually...
-
Vaughn Company sells two types of pumps. One is large and is for commercial use. The other is smaller and is used in residential swimming pools. The following inventory data is available for the...
-
To fund your dream around-the-world vacation, you plan to save $1,300 per year for the next 14 years starting one year from now. If you can earn an interest rate of 5.83 percent, how much will you...
-
On NSE (Indian stock exchange), shares of ICICI Bank trade for 935 rupees. If the spot exchange rate is USD 0.012, what is the no-arbitrage USD price of ICICI Bank ADR? Assume that transactions costs...

Study smarter with the SolutionInn App


