a. Define specific heat capacity of a substance. b. A mass of 20 g of ice at
Question:
a. Define specific heat capacity of a substance.
b. A mass of 20 g of ice at −15 °C is taken from a freezer and placed in a beaker containing 200 g of water at 26 °C. Data for ice and water are given in Table 19.5.
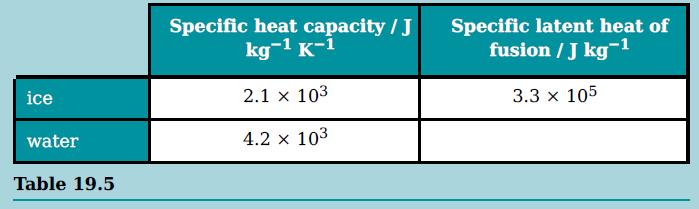
i. Calculate the amount of thermal energy (heat) needed to convert all the ice at −15 °C to water at 0 °C.
ii. Calculate the final temperature T of the water in the beaker assuming that the beaker has negligible mass.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:





