A well-insulated 1.2 kg block of iron is heated using a 50 W heater for 4.0 min.
Question:
A well-insulated 1.2 kg block of iron is heated using a 50 W heater for 4.0 min. The temperature of the block rises from 22 °C to 45 °C. Find the experimental value for the specific heat capacity of iron.
You will need to use data from Table 19.3 to answer these questions.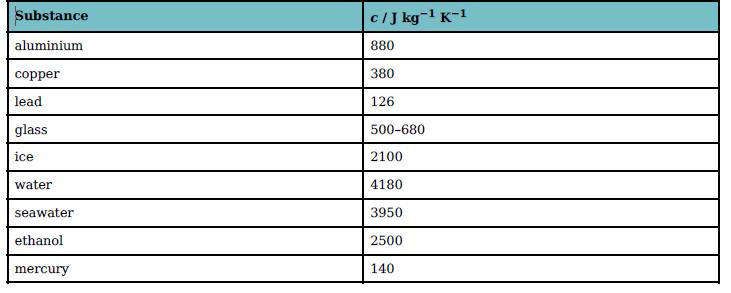
Substance cIJ kg-1 K-1 aluminium 880 copper 380 lead 126 glass 500-680 ice 2100 water 4180 seawater 3950 ethanol 2500 mercury 140
Step by Step Answer:
Given A well insulated block of iron has Massm 12kg which is heated with the help of a heater hav...View the full answer



Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Sciences questions
-
A large, cold (0.0C) block of iron is immersed in a tub of hot (100.0C) water. In the first 10.0 s, 41.86 kJ of heat are transferred, although the temperatures of the water and the iron do not change...
-
For this assessment, you will need to use the SAS Enterprise Guide or the Excel Calculators, the latter of which can be found in Resources. SAS users can reference the SAS tutorials located in the...
-
A 3 kg block of iron at 800oC is dropped into 50 kg of water in an insulated cooling tank. If the final temperature (T2) at equilibrium is 29.9oC, determine (a) The change in internal energy (U). (b)...
-
Discuss the differences between @classmethod , @staticmethod , and instance methods in Python.
-
Mei Li invested $350 at the end of each quarter at 3.2% compounded quarterly. At the end of five years, she was able to withdraw equal amounts at the end of each quarter for nine years. How much is...
-
Television channel operating profits vary from as high as 45 to 55 percent at MTV and Nickelodeon down to 12 to 18 percent at NBC and ABC. Provide a Porter Five Forces analysis of each type of...
-
What would happen to the NPV of the above project if the inflation rate was expected to be 4 percent in each of the next four years? You may use either LO8 Excel or CB to determine your answer.
-
Do males or females feel more tense or stressed out at work? A survey of employed adults conducted online by Harris Interactive on behalf of the American Psychological Association revealed the...
-
D&L Corporation's profit and loss statement showed a net income of 93/4% of revenue or $41,250. Twenty-one percent of net income was paid in corporation tax and 65% of the net income after tax was...
-
The proposed rates were not in the range the CEO expected given the pricing analysis. The CEO has asked the pricing actuary to verify the total projected loss cost excluding potential large storm...
-
a. A 500 W kettle contains 300 g of water at 20 C. Calculate the minimum time it would take to raise the temperature of the water to boiling point. b. The kettle is allowed to boil for 2 minutes....
-
The resistance of a thermistor at C is 2000 . At 100 C the resistance falls to 200 . When the thermistor is placed in water of constant temperature, its resistance is 620 . a. Assuming that the...
-
_____ bankruptcy option that allows the business to file a reorganization plan for a second chance
-
+ Given f(x) = x - 9 and g(x) = x+9, complete the following. (a) Find f(g(x)) and g(f(x)). (Simplify your answers completely.) f(g(x)) = g(f(x)) = (b) What does this tell us about the relationship...
-
Case Study - Rhonda Rhonda is a 28-year-old woman who has been referred to your agency by a local probation officer. Rhonda reported that she has "fired" three counselors in the past and most...
-
Calculating depreciationpartial periods LO2, 3 West Coast Tours runs boat tours along the west coast of British Columbia. On March 5, 2020, it purchased, with cash, a cruising boat for $936,000,...
-
Question 1. Write down the form of partial fractions needed to decompose the following: 482+2 (a) s32s24s 482+2 (c) s36s20 482 +2 - 4s8 (b) 8. 3 - 282 482+2 (d) s3 +2s2 - 2 Note: You are not being...
-
On December 31, 2022, Ace Hardware reported the following information on its balance sheet Accounts Receivable Allowance for Doubtful Accounts $900,000 $54,000 (credit) During 2023, the Company had...
-
On June 15, 2018, Sheridan filed his 2017 income tax return, paying a tax of $10,500. On October 5, 2019, he filed an amended 2017 return showing an additional $6,400 of tax, which he paid with the...
-
Kenneth Hubbard has prepared the following list of statements about managerial accounting and financial accounting. 1. Financial accounting focuses on providing information to internal users. 2....
-
When ethoxybenzene is treated with a mixture of nitric acid and sulfuric acid, two products are obtained each of which has the molecular formula C 8 H 9 NO 3 . (a) Draw the structure of each product....
-
The pressure dependence of G is quite different for gases and condensed phases. Calculate G m for the processes (C, solid, graphite, 1 bar, 298.15 K) (C, solid, graphite, 325 bar, 298.15 K) and (He,...
-
Many biological macromolecules undergo a transition called denaturation. Denaturation is a process whereby a structured, biologically active molecule, called the native form, unfolds or becomes...
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


