Use the binding energy graph (Figure 29.6) to suggest why fission is unlikely to occur with light
Question:
Use the binding energy graph (Figure 29.6) to suggest why fission is unlikely to occur with ‘light nuclei’ (A < 20) and why fusion is unlikely to occur for heavier nuclei (A > 40).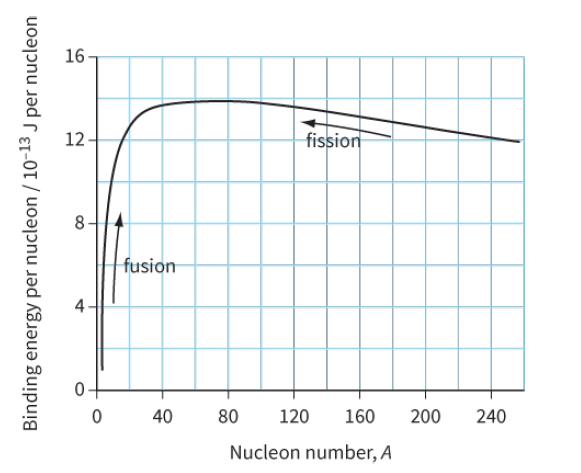
Transcribed Image Text:
16 12 fission fusion 40 80 120 160 200 240 Nucleon number, A -13 Binding energy per nucleon / 10- J per nucleon
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
Fission is unlikely to occur with light nuclei A 20 because the binding energy per nuc...View the full answer

Answered By

Akshay Shete
I have extensive experience as a tutor, both online and in-person. I have worked with students of all ages and abilities, and am skilled at adapting my teaching style to meet the needs of each individual student. I have a strong background in a variety of subjects, including math, science, and English, and am able to break down complex concepts in a way that is easy for students to understand. In addition to my subject matter expertise, I am also a patient and supportive teacher, and am committed to helping my students succeed. Whether I am working with a struggling student who needs extra help to catch up, or an advanced student looking to get ahead, I am able to provide the guidance and support they need to reach their goals. Overall, my hands-on experience as a tutor has prepared me to be a confident and effective teacher, and I am excited to use my skills to help students succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:
Students also viewed these Sciences questions
-
Suggest a reason why the relaxation times of BC nuclei are typically much longer than those of IH nuclei.
-
Light nuclei can be split. For example, a deuteron, which is a proton-neutron combination, can split into a separate proton and separate neutron. Does such a process yield energy or cost energy?...
-
Use Fig. 30-1 to estimate the total binding energy of (a) 238/92U, and (b) 84/36Kr.
-
1. Consider the market for local rides (taxis, Uber Lyft, and so on), which is highly competitive. Suppose that the market is initially unregulated, but that the government imposes a binding price...
-
You are the accountant for Peel Credit Union. The lawyer for a member has sent a cheque for $7345.64 in full settlement of the members loan balance including interest at 6.25% for 11 months. How much...
-
A large share of the world supply of diamonds comes from Russia and South Africa. Suppose that the marginal cost of mining diamonds is constant at $1,000 per diamond, and the demand for diamonds is...
-
3.5
-
Burglar Bob breaks into Vince Victims house. Bob steals a flat-screen TV and laptop and does a significant amount of damage to the property before he leaves. Fortunately, Vince has a state-of-the-art...
-
Diana and Ryan Workman were married on January 1 of last year. Diana has an eight-year-old son, Jorge, from her previous marriage. Ryan works as a computer programmer at Datafile Incorporated (DI)...
-
Terry Powell was a property owner in Marshall County, Kentucky. Tosh Farms General Partnership raised swine in barns on land within one mile of Terry Powells residence. Jimmy Tosh was the general...
-
The graph of count rate against time for a sample containing indium-116 is shown. a. Use the graph to determine the half-life of the isotope. b. Calculate the decay constant. 160 120 80 40 10 20 30...
-
The proportions of different isotopes in rocks can be used to date the rocks. The half-life of uranium-238 is 4.9 10 9 years. A sample has 99.2% of the proportion of this isotope compared with newly...
-
The following purchase related transactions for Hart, Inc., occurred during the month of June. Jun 3 Purchased $6,200 of merchandise, paid cash. 9 Purchased $450 of supplies on account from Hamilton...
-
Watch the clip from HBO's Chernobyl and respond to the questions below using complete sentences....
-
1) Every organization has a unique culture. As you move forward with a travel or per diem position, what steps will you take to learn about your assignment organization's culture? 2)Flexibility and...
-
See the right figure of the lifting and transporting equipment. During operation, while a mass of 1.5 x 104 kg was descending at a constant speed v = 15 m/min, the machine experienced a breakdown,...
-
PROBLEM 3 BABEY Company makes three products with the following characteristics: Product V jk jin Selling price per unit 10 15 20 Variable cost per unit 6 10 10 Machine hours per unit 2 4 10 The...
-
Diversified Semiconductors sells perishable electronic components. Some must be shipped and stored in reusable protective containers. Customers pay a deposit for each container received. The deposit...
-
The file StockComparison contains monthly adjusted stock prices for technology company Apple, Inc., and consumer-goods company Procter & Gamble (P&G) from 20132018. a. Develop a scatter diagram with...
-
Splitting hairs, if you shine a beam of colored light to a friend above in a high tower, will the color of light your friend receives be the same color you send? Explain.
-
Evaluate the commutator [ x, P 2 x ] by applying the operators to an arbitrary function f (x).
-
What is wrong with the following argument? We know that the functions n (x) = 2/a sin(n x/a) are eigenfunctions of the total energy operator for the particle in the infinitely deep box. We also know...
-
For linear operators A, B, and C, show that [A,BC] = [A,B]C + B[A,C].
-
Suppose the S&P 500 currently has a level of 960. One contract of S&P 500 index futures has a size of $250 S&P 500 index. You wish to hedge an $800,000-portfolio that has a beta of 1.2. (A)In order...
-
Exhibit 4.1 The balance sheet and income statement shown below are for Koski Inc. Note that the firm has no amortization charges, it does not lease any assets, none of its debt must be retired during...
-
Haley is 57 years of age. She is planning for future long-term care needs. She knows that yearly nursing home costs in her area are currently $69,000, with prices increased by 5 percent annually....

Study smarter with the SolutionInn App


