a. i. Which would be more readily attacked by an electrophile benzene or phenylamine? Explain your
Question:
a. i. Which would be more readily attacked by an electrophile – benzene or phenylamine? Explain your answer.
ii. Write a general equation to show the equation for the reaction of phenylamine with excess of an electrophile, represented as X+.
b. i. Why is the reaction of phenylamine to make the diazonium ion carried out below 10 °C?
ii. Write a balanced equation to show how nitrous acid is made for the reaction in part b i to take place.
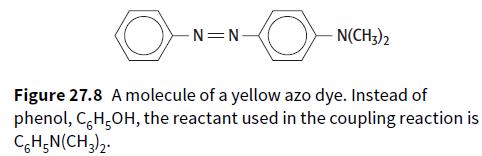
iii. Show the two steps that would be used to make the yellow dye shown in Figure 27.8, starting from phenylamine.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





