A sample of lactic acid (CH 3 CH(OH)COOH) was extracted from a natural source and found to
Question:
A sample of lactic acid (CH3CH(OH)COOH) was extracted from a natural source and found to be optically active. It was then subjected to two reactions, as shown below.
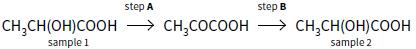
Sample 1 was optically active but sample 2 was not optically active.
a. i. Give the systematic name for lactic acid.
ii. The structure of one optical isomer of the lactic acid is:
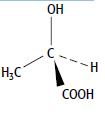
Draw the other optical isomer.
iii. Explain why lactic acid can form optical isomers.
b. i. Give the reagents and conditions necessary for step A.
ii. Give the balanced equation for the reaction.
c. i. Give the reagents and conditions necessary for step B.
ii. Give the balanced equation for the reaction.
d. i. Give the mechanism for step B. The first step involves nucleophilic attack on the carbon of the ketone group by an H– ion from NaBH4.
ii. Explain why sample 2 does not show any optical activity – it does not rotate plane-polarised light.
e. i. Explain why lactic acid can be polymerised.
ii. State the type of polymerisation reaction that this an example of.
iii. Draw the repeat unit of poly(lactic acid).
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





