Compound D has the composition 77.8% carbon, 7.41% hydrogen and 14.8% oxygen. It mass and 1H NMR
Question:
Compound D has the composition 77.8% carbon, 7.41% hydrogen and 14.8% oxygen. It mass and 1H NMR spectra are shown below.
a.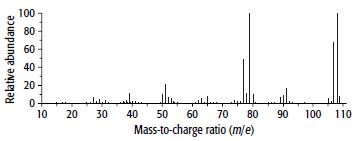
b.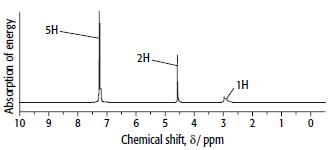
a. Calculate the empirical formula of D.
b. From the mass spectrum, find the molecular mass of D (ignoring the 13C peak) and hence it molecular formula.
c. i. Draw displayed formulae for five possible isomers of D that contain a benzene ring.
ii. Use the 1H NMR spectrum of D to decide which isomer is D.
iii. Explain your reasoning.
d. Explain the main features of the NMR spectrum of D.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





