Gaseous hydrogen and gaseous iodine react together to form hydrogen iodide. H 2 + I 2
Question:
Gaseous hydrogen and gaseous iodine react together to form hydrogen iodide.
H2 + I2 ⇋ 2HI
a. The graph shows how the amount of hydrogen iodide varies with time in a 1.00 dm3 container. The initial amounts of hydrogen and iodine were 1.00mol H2 and 1.00mol I2.
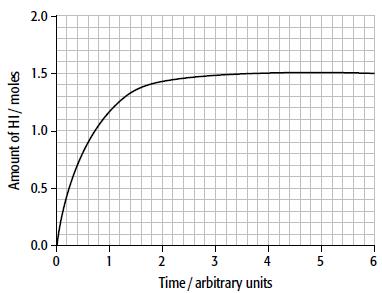
Draw a similar graph to show how the number of moles of hydrogen varies with time.
b. Calculate the number of moles of iodine present at equilibrium.
c. i. Write the equilibrium expression for Kc for the reaction between gaseous hydrogen and iodine.
ii. Calculate the value of Kc and give the units.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





