Use Table 21.3 to identify: a. Those indicators which could be used for a strong acidstrong base
Question:
Use Table 21.3 to identify:
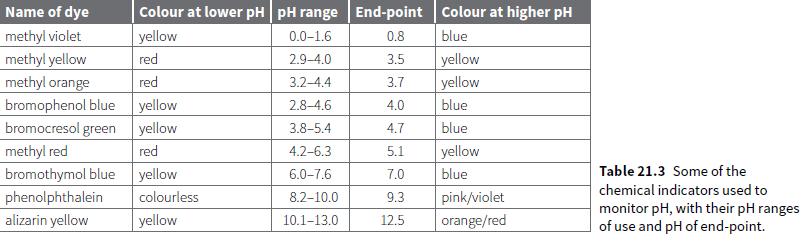
a. Those indicators which could be used for a strong acid–strong base titration like the one in Figure 21.8.
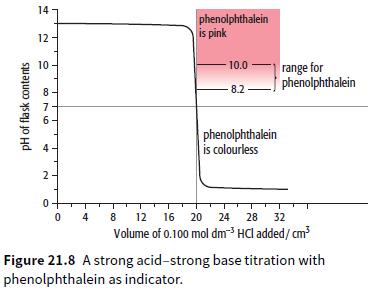
b. Those indicators that could not be used.
Transcribed Image Text:
Name of dye Colour at lower pH pH range End-point Colour at higher pH methyl violet yellow 0.0-1.6 0.8 blue methyl yellow methyl orange bromophenol blue yellow bromocresol green yellow red 2.9-4.0 3.5 yellow red 3.2-4.4 3.7 yellow 2.8-4.6 4.0 blue 3.8-5.4 4.7 blue methyl red red 4.2-6.3 5.1 yellow bromothymol blue yellow 6.0-7.6 blue Table 21.3 Some of the 7.0 chemical indicators used to phenolphthalein colourless 8.2-10.0 9.3 pink/violet monitor pH, with their pH ranges of use and pH of end-point. alizarin yellow yellow 10.1-13.0 12.5 orange/red
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 55% (9 reviews)
a Strong acid and strong base give its end point at near neutral ...View the full answer

Answered By

Dharshini P
I am a master's in chemistry from the University of Delhi and Since 2015 I am teaching in both offline and online modes. In total, I have 7 years of teaching experience.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
Which of the indicators in Fig. could be used for doing the titrations in Exercises 61 and 63? Fig Pheadl Red
-
Which of the indicators in Fig could be used for doing the titrations in Exercises 62 and 64? Fig Pheadl Red
-
Which of the indicators in Fig. could be used for doing the titrations in Exercises 65 and 67? Fig Pheadl Red
-
Find a minimum spanning tree for the following graph using all 3 algorithms 13 17 22- 20 15, a. Adding the shortest edge first b. Deleting the longest edge first c. Growing a tree from the node D
-
Sketch the areas under the standard normal curve over the indicated intervals and find the specified areas. To the left of z = 0
-
Prior to the invention of the printing press in the mid- 1400s, the process of producing fine books, called illuminated manuscripts, was strictly manual and performed by skilled craftsmen. A scribe...
-
Under what circumstances is it ethically appropriate to use coercive power? When should managers not use coercive power to deal with problems in organizations?p. 408
-
Orange Corp. has two divisions: Fruit and Flower. The following information for the past year is available for each division: Orange has established a hurdle rate of 12 percent. Required: 1. Compute...
-
Silverado Corporation produces one product, electric gnomes for gardens, and uses process costing (weighted average method). The work-in-process inventory on October 1 consisted of 3,000 gnomes with...
-
A local group of scouts has been collecting old aluminum cans for recycling. The group has already collected 12,000 lb of cans, for which they could currently receive $4 per hundred pounds. The group...
-
A saturated solution of copper(I) sulfide, Cu 2 S, contains 1.91 10 12 g of Cu 2 S dissolved in 1 dm 3 of water. (A r values: Cu = 63.5, S = 32.1) a. Write an equilibrium expression for the...
-
a. What is the pH of 0.25 mol dm 3 HCl(aq)? b. What is the pH of 0.0500 mol dm 3 sodium hydroxide? (Kw = 1.00 1014 mol 2 dm 6 ) c. The graph shows how the pH changes when 0.100 mol dm3 ethanoic acid...
-
Explain how long-term contractual relationships with suppliers can reduce the ac- quisition cost of materials. LO1
-
ProForm acquired 70 percent of ClipRite on June 30, 2020, for $1,470,000 in cash. Based on ClipRite's acquisition-date fair value, an unrecorded intangible of $600,000 was recognized and is being...
-
Consider the function f(x) = e^(cos(x)) USING MATLAB a) Write code that will approximate the definite integral of f(x) over [0, 1] using Simpson's rule with 100 evenly spaced subintervals. Compare...
-
What type of research funding method involves shared responsibility for conducting a research project and may grant the funder the authority to withdraw funding if the researcher does not adhere to...
-
Chapter 6 Assignment i 5 Problem 6-52 (LO 6-4) 12.5 points Jordan took a business trip from New York to Denver. She spent two days in travel, conducted business for nine days, and visited friends for...
-
need help completing my one-month project. anyone willing help me. Ellipses Corp One Month Project Ellipses Corp is a small business that operates in Herndon, VA. The company is located at10 Period...
-
TopChop sells hairstyling franchises. TopChop receives $50,000 from a new franchisee for providing initial training, equipment, and furnishings that have a stand-alone selling price of $50,000....
-
On the basis of the details of the following fixed asset account, indicate the items to be reported on the statement of cashflows: ACCOUNT Land ACCOUNT NO. Balance Date Item Debit Credit Debit Credit...
-
Draw the structure of the cyclic compound that is produced when acetone is treated with 1,3 propanedithiol in the presence of an acid catalyst. SH 1,3-propanedithiol HS Acetone
-
Predict the major product for each of the following reactions: (a) (b) (c) (d) 1) LAH 2) H20 H NABH4,
-
When 2 moles of benzaldehyde are treated with sodium hydroxide, a reaction occurs in which 1 mole of benzaldehyde is oxidized (giving benzoic acid) while the other mole of benzaldehyde is reduced...
-
Question 24 Not yet answered Marked out of 1.00 P Flag question Muscat LLC's current assets and current liabilities are OMR 258,000 and OMR 192,000, respectively. In the year 2020, the company earned...
-
Question 24 Miami Company sold merchandise for which it received $710,400, including sales and excise taxes. All of the firms sales are subject to a 6% sales tax but only 50% of sales are subject to...
-
f the IRS intends to close a Taxpayer Assistance Center, they must notify the public at least _____ days in advance of the closure date. 14 30 60 90

Study smarter with the SolutionInn App


