Use the information in the table to deduce the number of electrons and neutrons in a neutral
Question:
Use the information in the table to deduce the number of electrons and neutrons in a neutral atom of:
a. Vanadium
b. Strontium
c. Phosphorus.
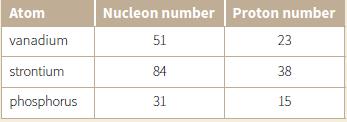
Transcribed Image Text:
Atom Nucleon number Proton number vanadium 51 23 strontium 84 38 phosphorus 31 15
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
element Nucleon Number or Mass number ...View the full answer

Answered By

Konda Venkatesham
I'm having 32+ years of teaching experience in dealing chemistry for high school, college levels and entrance exams like EAMCET, EE, and NEET.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
In each part, use the information in the table to determine whether the linear system Ax = b is consistent. If so, state the number of parameters in its general solution. 3x3 3x3 3x3 5x9 5x9 4x4 6x2...
-
Use the information in Table 2.5 to predict the standard reaction enthalpy of2 H2 (g) + 02(g) 2 H2O (1) at 100C from its value at 25C.
-
Use the information in Table 18.2 on page 602 to calculate the total federal income tax paid, the marginal tax rate, and the average tax rate for people with the following incomes. (For simplicity,...
-
Express the following sum in closed form. 2 k 2 (4 + 3 - 4 ) = n k 1
-
Determine the mass of each substance. a. Na b. B2O3 c. S2Cl2
-
The distribution system for the Herman Company consists of three plants, two warehouses, and four customers. Plant capacities and shipping costs per unit (in $) from each plant to each warehouse are...
-
9. Instead of producing the tables, the company could rent its factory space for $50,000 per year. McFarland, Inc., is a merchandiser that provided the following information: Number of units sold....
-
Christy Albright and Dan Ralls formed the Charter Company on 11/30/2012, and chose a tax year ending on 11/30. Charter was formed to operate a restaurant (at 7848 Pesca Dr., San Francisco, CA 94123)...
-
Question 3 (30 marks) The comparative statement of financial positions and statement of profit or loss of Chloe Electronics Limited are as follows: Chloe Electronics Limited Statement of financial...
-
For each of the following scenarios, estimate how much value an acquisition will create, how much of that value will be appropriated by each of the bidding firms, and how much of that value will be...
-
Zirconium, Zr, and hafnium, Hf, are metals. An isotope of zirconium has 40 protons and 91 nucleons. a. i. Write the isotopic symbol for this isotope of zirconium. ii. How many neutrons are present in...
-
A beam of electrons is passing close to a highly negatively charged plate. When the electrons pass close to the plate, they are deflected (bent) away from the plate. a. What deflection would you...
-
On 1 June 2022 Sydney Ltd enters into a firm commitment with SanFran Co. to buy US$1 000 000 of6 inventory. The inventory will be transferred to Sydney Ltd (making Sydney Ltd therefore liable for the...
-
If the dose rate from a sample of Ga-67 is 0.052 mSv per hour at a distance of 1.1 m, then what would be dose rate at 3.5 m ?
-
A 1.6x10^9 p/s point source of Po210-Be source of 4.5 MeV is stored behind a X cm of paraffin, the dose equivalent rate is not to exceed 0.10 mSvh-1h at a distance of 1m. What is the X cm needed to...
-
X 10 Let A = -9 y 7 4 Z 210 If the kernel of A contains the vector what are x, y, and z? -2
-
8-22. E.O.Q., Carrying cost = Storing cost + Interest. Following data are available with respect to a certain material. Annual requirement.......... Cost to place an order.. Annual interest rate. _...
-
A new company started production. Job 1 was completed, and Job 2 remains in production. Here is the information from the job cost sheets from their first and only jobs so far: Job 1 Hours Total Cost...
-
Classified Electronics has an unfunded retiree health care plan. Each of the companys three employees has been with the firm since its inception at the beginning of 2020. As of the end of 2021, the...
-
Imagine you are the HR manager at a company, and a female employee came to you upset because she felt a male coworker was creating a hostile work environment by repeatedly asking her out on dates...
-
The barrier to rotation of bromoethane is 15 kJ/mol. Based on this information, determine the energy cost associated with the eclipsing interaction between a bromine atom and a hydrogen atom.
-
Menthol, isolated from various mint oils, is used in the treatment of minor throat irritation. Draw both chair conformations of menthol, and indicate which conformation is lower in energy. Menthol
-
Draw both chair conformations for each of the following compounds. In each case, identify the more stable chair conformation: (a) Methylcyclohexane (b) Trans-1,2-Diisopropylcyclohexane (c)...
-
You have just been hired as a new management trainee by Earrings Unlimited, a distributor of earrings to various retail outlets located in shopping malls across the country. In the past, the company...
-
Difference between Operating Leverage and Financial Leverage
-
bpmn diagram for misc purchases

Study smarter with the SolutionInn App


