An equimolar mixture of nitrogen and acetylene enters a steady-flow reactor at 25C and 1 bar of
Question:
An equimolar mixture of nitrogen and acetylene enters a steady-flow reactor at 25°C and 1 bar of pressure. The only reaction occurring is
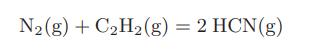
The product leaves the reactor at 600°C and contains 24.2 percent mole fraction of HCN. How much heat is supplied to the reactor per mole of HCN?
Transcribed Image Text:
N₂(g) + C₂H₂(g) = 2 HCN(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate the heat supplied to the reactor per mole of HCN we need to perform an energy balance on the reactor The energy balance states that the t...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
An equimolar mixture of nitrogen and acetylene enters a steady-flow reactor at 25(C and atmospheric pressure. The only reaction occurring is: N2(g) + C2H2 ( 2HCN(g). The product gases leave the...
-
An equimolar mixture of oxygen and nitrogen enters a compressor operating at steady state at 10 bar, 220 K with a mass flow rate (m) of 1 kg/s. The mixture exits the compressor at 60 bar, 400 K with...
-
Jacky is single and will turn 24 this year. He doesn't have any dependents. He does not have any group coverage. He drives a motorcycle to work every day. From your perspective which insurance policy...
-
Pritchett Company reported the following year-end data: Cash $ 25,000 8,000 Short-term investments Accounts receivable (current) Inventory 19,500 27,500 Prepaid (current) assets 11,000 Total current...
-
Yolanda Christophe filed a bankruptcy petition under Chapter 13. Her scheduled debts consist of $11,100 of secured debt, $9,300 owed on an unsecured student loan, and $6,960 of other unsecured debt....
-
Terminations for ________________ often result from a problem with the projects cost, schedule, or performance. a. convenience b. completion c. default d. confidence
-
33. Compare and contrast the tests for accruing income and those for accruing deductions for tax purposes.
-
Prado Roberts Manufacturing is a medium-sized company with regional offices in several western states and manufacturing facilities in both California and Nevada. The company performs most of its...
-
Quatro Con bonds dated January 1, 2019, with a par value of $80,000. The bonds al contract rate is 12% and interest bald semiannually on June 30 and December 3 The bonds mature in three years. The...
-
STYLIZE GUI USING CSS AND PRESENT APPLICATION TO STAKEHOLDERS Overview The visual appearance (look and feel) of an application greatly affects its acceptance and usefulness. You need to ensure that...
-
The following data are available for the isothermal heat of mixing of trichloromethane (1) and ethanol (2) at 30C [reference: J. P. Shatas, M. M. Abbott, and H. C. Van Ness, J. Chem. Eng. Data, 20,...
-
Using the data below, calculate the partial molar enthalpies of 1-propanol and water as a function of composition at both 25C and 50C. data: V. P. Belousov, Vent. Leningrad Univ. Fiz., Khim, 16(1),...
-
A bug on the surface of a pond is observed to move up and down a total vertical distance of 6.0 cm, from the lowest to the highest point, as a wave passes. If the ripples decrease to 4.5cm, by what...
-
Determine the mean number of credit cards based on the raw data. (b) Determine the standard deviation number of credit cards based on the raw data. (c) Determine a probability distribution for the...
-
B Harry is a county Department of Social Services worker whose clients consist primarily of poor, female-headed families receiving public assistance. During one of his meetings with Dora, a single...
-
1 A, Weakly coupled carts (20 points) m m2 Figure 1: A system of two masses and three springs. A symmetric two degree of freedom system consists of two identical rigid masses m = m = m pictured in...
-
A farmer has an acre of specialty vegetables and is preparing for the summer harvest. Historically, this acre has yielded an average of 2,100 lbs of product with a standard deviation of 950 lbs. A...
-
Solve 3x 82+22 = (4).
-
a. Determine VL, IL, IZ, and IR for the network Fig. 2.181 if RL = 180 Ω. b. Repeat part (a) if RL = 470 H. c. Determine the value of RL, that will establish maximum power conditions for...
-
(a) Given a mean free path = 0.4 nm and a mean speed vav = 1.17 105 m/s for the current flow in copper at a temperature of 300 K, calculate the classical value for the resistivity of copper. (b)...
-
Develop a table that lists advantages and disadvantages of the three design concepts for a mousetrap-powered vehicle in the case study in Section 2.4. Discuss the trade-offs between front and rear...
-
Three concepts for the drive mechanism in a mousetrap-powered vehicle are described in Section 2.4. Develop another concept, prepare several sketches, and write a brief description of it.
-
Express your weight in the units of pounds and newtons, and your mass in the units of slugs and kilograms.
-
True or False: Capitalization rate is used in valuing companies and ignores the effect of debt
-
Question 9 The following information is available for Astrid Ltd and Duncast Ltd. Astrid 15 000 Duncast 15 000 Units produced and sold Rm Rm Revenues 112.5 112.5 55.0 15.0 Variable costs Fixed costs...
-
What is the quoted price of a bond maturing in 12 years with a coupon rate of 9 percent, paid semiannually, that has a YTM of 13 percent? (Please round to the nearest hundredth)

Study smarter with the SolutionInn App


