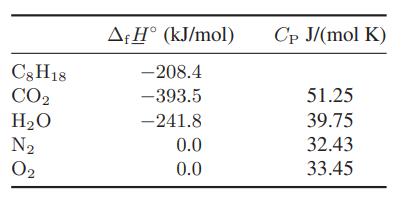
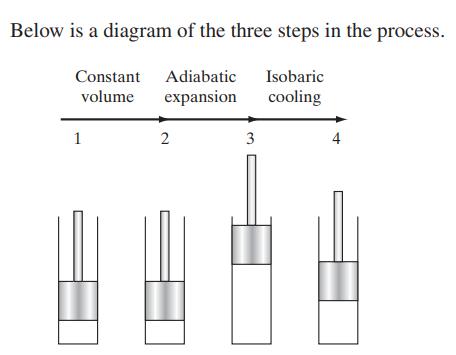
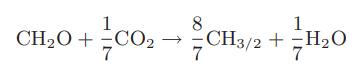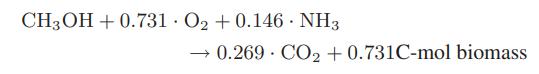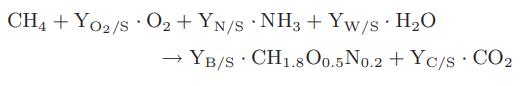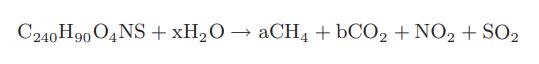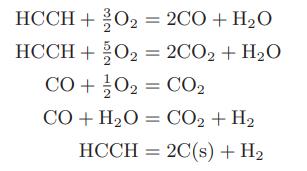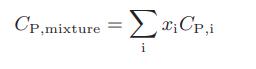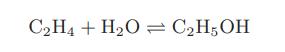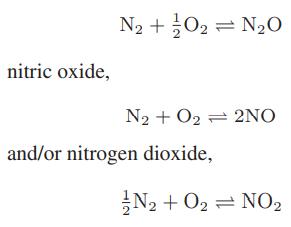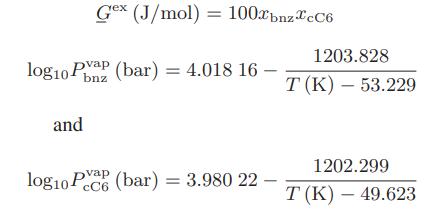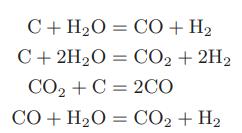Chemical Biochemical And Engineering Thermodynamics 5th Edition Stanley I. Sandler - Solutions
Unlock the full potential of "Chemical Biochemical and Engineering Thermodynamics, 5th Edition" by Stanley I. Sandler with our comprehensive solutions. Access an extensive collection of solved problems and answers key in our online solution manual. Discover detailed chapter solutions, step-by-step answers, and a complete test bank tailored for instructors and students alike. Our textbook resources include a free download of the solutions PDF, providing clear and concise questions and answers for enhanced understanding. Whether you seek an instructor manual or thorough textbook guidance, our platform offers everything you need to excel in this subject.
![]()
![]() New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
New Semester Started
Get 50% OFF
Study Help!
--h --m --s
Claim Now
![]()
![]()



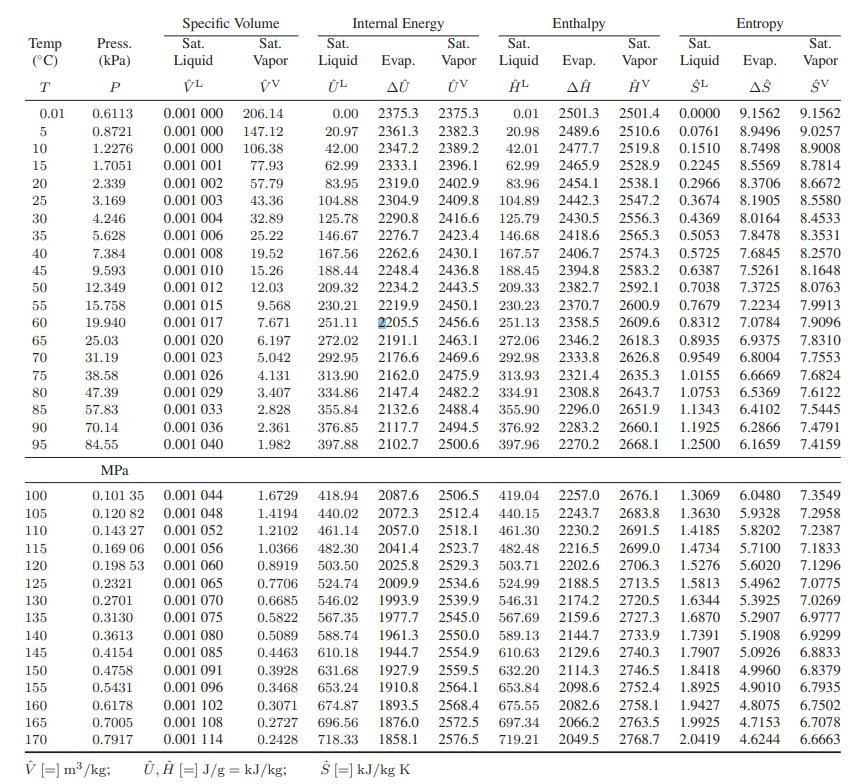
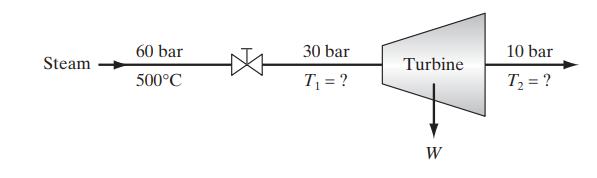
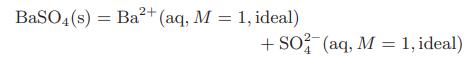

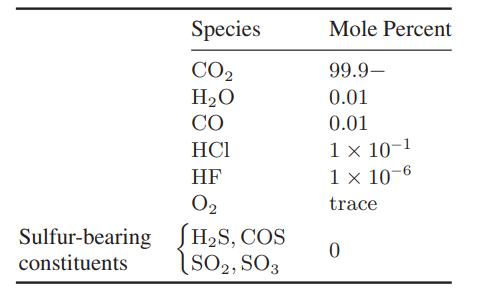
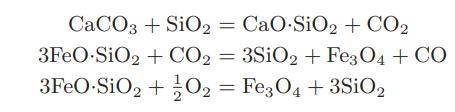
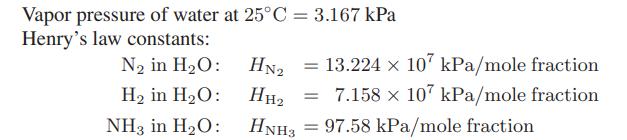
![[M] MGdnHCl = 0 MGdnHCl = 2.5 2.5 Murea = 0 Murea = 6.5 Aunt G (kcal/mol) 7.9 0 9.3 0](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/9/9/1076558816399e3f1700299105419.jpg)
![NaCl [M] Tunf (C) AunfH (kcal/mol) AunfG (kcal/mol) 0.1 87.0 106.9 1.1 8.9 0.3 0.5 87.0 103.5 1.1 8.4 0.3](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/2/9/9/16065588198935741700299158329.jpg)