Redo Illustration 7.5-3 with the SoaveRedlichKwong equation of state. Compare the results obtained with those for the
Question:
Redo Illustration 7.5-3 with the Soave–RedlichKwong equation of state. Compare the results obtained with those for the Peng-Robinson equation in the illustration. Also, repeat the calculations for the Soave– Redlich-Kwong equation but with the simplifying assumption that the α function of Eqs. 6.7-6 and 6.7-8 is a constant and equal to unity, rather than a function of temperature.
Illustration 7.5-3
Vapor Pressure Calculations for Water with the Peng-Robinson Equation of State
a. Compare the predictions for the vapor pressure of water from the Peng-Robinson equation of state with generalized coefficients with data in the saturated steam tables.
b. Use the PRSV equation of state with Eqs. 7.5-1 and 7.5-2 with κ1 = −0.0665 to calculate the vapor pressure of water, and compare the results with data in the steam tables.
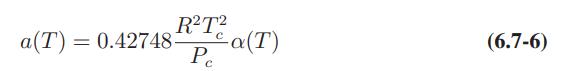
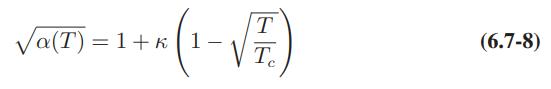
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





