Succinic acid, which has the structure HOOCCH 2 CH 2 COOH and on a C-mole basis is
Question:
Succinic acid, which has the structure HOOC—CH2—CH2—COOH and on a C-mole basis is represented by CH3/2O, is useful as a starting material in the manufacture of solvents and polymers. It is typically made by the catalytic oxidation of butane. However, by that route only about 40 percent of the carbon initially present in the butane is converted to succinic acid. It has been suggested instead to use glucose as a starting material and a biochemical pathway with the following proposed reaction stoichiometry:
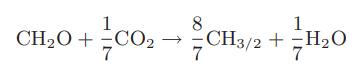
a. Is this reaction stoichiometry possible?
b. Determine the maximum kg of succinic acid that can be produced per kg of glucose.
c. Estimate the heat released per C-mole of glucose consumed. d. What fraction of the Gibbs energy of the glucose appears in the succinic acid?
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





