Using the information in Problems 7.13 and 8.20, estimate the heat of vaporization for the first bit
Question:
Using the information in Problems 7.13 and 8.20, estimate the heat of vaporization for the first bit of ethanol from ethanol-water solutions containing 25, 50, and 75 mol % ethanol and from a solution infinitely dilute in ethanol. How do these heats of vaporization compare with that for pure ethanol computed in Problem 7.13? Why is there a difference between the various heats of vaporization?
Problems 7.13.
a. The following data have been reported for the vapor pressure of ethanol as a function of temperature.
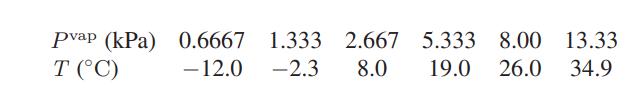 Use these data to calculate the heat of vaporization of ethanol at 17.33°C.
Use these data to calculate the heat of vaporization of ethanol at 17.33°C.
b. Ackermann and Rauh have measured the vapor pressure of liquid plutonium using a clever mass effusion technique. Some of their results are given here:
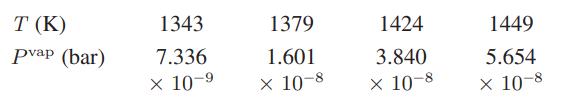
Estimate the heat of vaporization of liquid plutonium at 1400 K.
Problems 8.20.
A partial molar property of a component in a mixture may be either greater than or less than the corresponding pure-component molar property. Furthermore, the partial molar property may vary with composition in a complicated way. Show this to be the case by computing (a) the partial molar volumes and (b) the partial molar enthalpies of ethanol and water in an ethanol-water mixture. (The data that follow are from Volumes 3 and 5 of the International Critical Tables, McGraw-Hill, New York, 1929.)
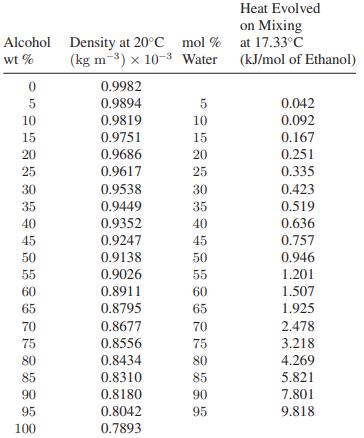
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





