a. The following data have been reported for the vapor pressure of ethanol as a function of
Question:
a. The following data have been reported for the vapor pressure of ethanol as a function of temperature.
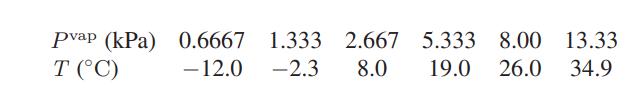 Use these data to calculate the heat of vaporization of ethanol at 17.33°C.
Use these data to calculate the heat of vaporization of ethanol at 17.33°C.
b. Ackermann and Rauh have measured the vapor pressure of liquid plutonium using a clever mass effusion technique. Some of their results are given here:
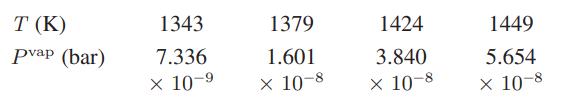
Estimate the heat of vaporization of liquid plutonium at 1400 K.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





