The following data are available for water: a. Compute the triple-point temperature and pressure of water. b.
Question:
The following data are available for water:
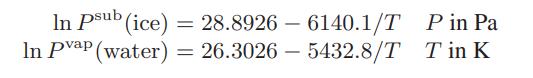
a. Compute the triple-point temperature and pressure of water.
b. Compute the heat of vaporization, the heat of sublimation, and the heat of fusion of water at its triple point.
Transcribed Image Text:
In Psub (ice) In Pvap (water) = 28.89266140.1/T = 26.3026-5432.8/T P in Pa Tin K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
The image you sent shows the following data for water Property Equation ln Psub ice 288926 61401T ln pvap water 263026 54328T where Psub ice is the va...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
The following data are available for carbon tetrachloride: a. Compute the heat of vaporization of carbon tetrachloride at 200C using only these data. b. Derive the following expression, which can be...
-
The triple point of iodine, I 2 , occurs at 112.9C and 11.57 kPa. The heat of fusion at the triple point is 15.27 kJ/mol, and the following vapor pressure data are available for solid iodine:...
-
The following data are available for Haul-It-Away Truckers: 1. Compute the predetermined overhead rate for each of the two years, if based on (a) Direct labor hours, (b) Number of moving jobs, and...
-
Use the following information for this question: Taxable income Marginal tax rate 15% 25% 34% 39% 34% 35% S S 0-S 50,000 75,000 50,000-$ S 75,000 $100,000 $ 100,000-S 335,000 S 335,000-$10,000,000...
-
Farthing is a director and vice president of Garp, Inc., whose common stock is listed on the New York Stock Exchange. Farthing engaged in the following transactions in the same calendar year: on...
-
What is the divisions profit margin ratio? a. 400% b. 20% c. 25% d. 80% Assume the Residential Division of Kipper Faucets had the following results last year: Net sales Operating income Average total...
-
Which of the four mechanisms outlined above do you think is usually most efficient for setting the following? LO7 1 Base pay 2 Annual cost of living increases 3 Executive remuneration packages 4...
-
Evans Ltd. publishes a monthly newsletter for retail marketing managers and requires its subscribers to pay $50 in advance for a one-year subscription. During the month of September 2010, Evans Ltd....
-
Production and sales estimates for June are as follows: Estimated inventory (units), June 1 8,000 Desired inventory (units), June 30 9,000 Expected sales volume (units): Area X 4,000 Area Y 10,000...
-
Brian Smith, network administrator at Advanced Energy Technology (AET), was given the responsibility of implementing the migration of a large data center to a new office location. Careful planning...
-
a. The following data have been reported for the vapor pressure of ethanol as a function of temperature. Use these data to calculate the heat of vaporization of ethanol at 17.33C. b. Ackermann and...
-
Prove that C P C V for any fluid, and identify those conditions for which C P = C V .
-
Who is typically more brand loyala consumer of goods or a consumer of services? Please explain.
-
Draw an original market equilibrium that describes the state of the market before the given scenario occurs. Clearly label both axis, label each a single supply curve and a single demand curve, and...
-
Analyze tools and/or metrics that a leader or manager should use to ensure that they are aligned and working together. Evaluate leadership strategies that could be employed to foster a positive...
-
Mexico has two main government programs that transfer income to rural households. PROCAMPO , which pays a set amount per acre to farmers who grew basic grains in a base year prior to the elimination...
-
Identify at least two business systems that support the development of effective work relationships Briefly explain how each system supports the development of effective work relationships.
-
Power and Influence Personal Plan - How will you navigate the realms of power and influence? Why is this personal plan important for you? What do you want to achieve? do a table with SMART goals -...
-
What peptide would be synthesized from the following DNA sequence? 5TTACCGACTGGTCACTCCCAT3
-
How has the globalization of firms affected the diversity of their employees? Why has increased diversity put an additional burden on accounting systems?
-
Use MATLAB to nd the products AB and BA for the following matrices: 11 5 -7 -8 B = -9 -4 6
-
Given the matrices Use MATLAB to a. Verify the associative property A(B + C) = AB + AC b. Verify the distributive property (AB)C = A(BC) 4 -2 1 6 9 -4 -4 -5 2 A = 8 -5 B = 7 5 3 C = 10 6. 1 7 9. 10...
-
The following tables show the costs associated with a certain product and the production volume for the four quarters of the business year. Use MATLAB to nd (a) The quarterly costs for materials,...
-
A company has $60 billion of sales and $3 billion of net income. It total assets are $ 30 billion. The companys total assets equal total invested capital, and its capital consists of half debt and...
-
Project ABC Initial End-of-Year Investment Cash Flows for years 1-3, respectively $47,000 $20,000 30,000 24,000 WACC = 14% What is the NPV? (Please round to the nearest dollar and do not enter the...
-
A semi-annual coupon bond has 15 years left to maturity. Its coupon rate is 6.5%. If you require an annual rate of return at 7% for your investment. What is this bond's intrinsic value to you? Please...

Study smarter with the SolutionInn App


