Consider the Hilsch-Ranque vortex tube discussed in Illustration 4.5-6. Starting with air at 4 bar and 25C,
Question:
Consider the Hilsch-Ranque vortex tube discussed in Illustration 4.5-6. Starting with air at 4 bar and 25°C, an exhaust pressure of 1.013 bar, and that half the air that enters the tube will be withdrawn at the higher temperature, what is the maximum temperature difference that can be obtained? Treat air as an ideal gas with C∗P = 29.3 ![]()
Illustration 4.5-6
Showing That the Energy and Entropy Balances Can Be Used to Determine Whether a Process Is Possible
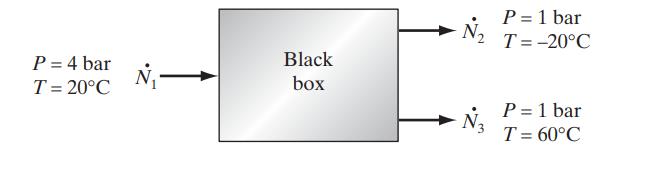
An engineer claims to have invented a steady-flow device that will take air at 4 bar and 20°C and separate it into two streams of equal mass, one at 1 bar and −20°C and the second at 1 bar and 60°C. Furthermore, the inventor states that his device operates adiabatically and does not require (or produce) work. Is such a device possible? [Air can be assumed to be an ideal gas with a constant heat capacity of C∗P = 29.3 J/(mol K)].
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





