Question: At high temperaturesfor example, in a combustion processnitrogen and oxygen in air can react to form nitrous oxide, Starting with air (79 mol % nitrogen
At high temperatures—for example, in a combustion process—nitrogen and oxygen in air can react to form nitrous oxide,
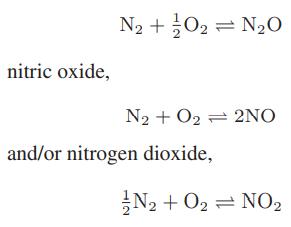
Starting with air (79 mol % nitrogen and 21 mol % oxygen), compute the equilibrium concentrations of all the oxides of nitrogen at atmospheric pressure over the temperature range from 1000 to 2000 K. [The oxides of nitrogen are referred to collectively as NOx compounds, and are smog-forming air pollutants.]
nitric oxide, N + O2 = NO N2 + O2 2NO and/or nitrogen dioxide, N +02= NO
Step by Step Solution
3.36 Rating (146 Votes )
There are 3 Steps involved in it
The following chemical equations show the formation of the oxides of nitrogen N2g O2g 2NOg Kc 120 x ... View full answer

Get step-by-step solutions from verified subject matter experts


