For the study of the oxidation of methane, an engineer devises the following set of possible reactions:
Question:
For the study of the oxidation of methane, an engineer devises the following set of possible reactions:
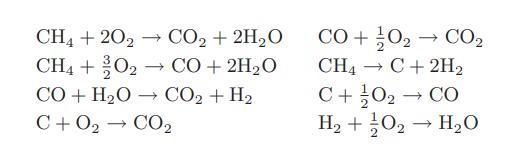
How many independent chemical reactions are there in this system?
Transcribed Image Text:
CH4 + 202 CO₂ + 2H₂O CH4 + 02 - → CO + 2H₂O CO+H2O → CO2+H2 C+02 → CO2 CO + 0₂ → CO₂2 CH₁ C+ 2H₂ → C+0₂ → CO H₂ + O₂ → H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
There are two independent chemical reactions in the given system The reactions are CH4 2O2 CO2 2H2O ...View the full answer

Answered By

Navashree Ghosh
I believe in quality work and customer satisfaction. So, I can assure you that you will get quality work from me when you hire me. Let's work together and build a long-term association.
4.90+
82+ Reviews
116+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Give two "real-world" examples (with the stochastic matrix and what it is modeling) of Markov chain models which contain: (a) Periodic classes (groups) of states (b) An ergodic system
-
In a review of the research on workplace diversity, Beryl Nelson, a former software-engineering manager at Google, referred to several studies showing that teams whose members are...
-
How many words are there in this book?
-
What is a safe edge on a file?
-
A debtor went through bankruptcy under Chapter 7 and received his discharge. Which of the following debts were completely discharged, and which will remain as future debts against him? (a) A claim of...
-
You are the project manager in charge of renovating a large apartment building, and your team has decided to outsource the installation of a new septic system. Do you put out an RFQ or RFP to...
-
16. How does a casualty loss on a business asset differ when the asset is stolen as opposed to destroyed in a fire?
-
Answer each of the following questions. a. What single investment made today, earning 12% annual interest, will be worth $6,000 at the end of 6 years? b. What is the present value of $6,000 to be...
-
Blue Company purchased a new machine on September 1, 2022, at a cost of $141.000. The company estimated that the machine will have a salvage value of $14.000. The machine is expected to be used for...
-
Balance sheets and income statements for Nike, Inc., and Adidas Group follow. Refer to these financial statements to answer the requirements. REQUIRED: a. Compute return on equity (ROE), return on...
-
Derive the expression for the fugacity coefficient of the SoaveRedlich-Kwong equation of state (Eq. 4.4-1b) with the van der Waals one-fluid mixing and combining rules of Eqs. 9.4-8 and 9.4-9. amix =...
-
Derive the following two independent equations for a second-order phase transition: These equations, which are analogues of the Clapeyron equation, are sometimes referred to as the Ehrenfest...
-
The purpose of this exercise is to demonstrate the matching principle in a familiar setting. Assume that you own a car that you drive about 15,000 miles each year. a. List the various costs to you...
-
On September 22, 2024, a flood destroyed the entire merchandise inventory on hand in a warehouse owned by the Rocklin Sporting Goods Company. The following information is available from the records...
-
A wound DC motor is connected in both a shunt and a series configuration. Assume generic resistance and inductance parameters Ra, Rf, La, Lf, let the field magnetization constant be kf and the...
-
Supermart Food Stores (SFS) has experienced net operating losses in its frozen food products line in the last few periods. Management believes that the store can improve its profitability if SFS...
-
Current Attempt in Progress The adjusted trial balance of Anthony Co. for the year ending December 31, 2025, contains the following. Anthony Co. Adjusted Trial Balance December 31, 2025 Debit Credit...
-
The coefficient of performance (COP) for a heat pump used as a heater (of a house, for example) is defined as 0=-QH/W, the ratio of the total heat flow -QH into the hot place (the house) to the work...
-
Determine vo for each network of Fig. 2.172 for the input shown. 10 V Si 2,2 k Si 5 V 1.2 k 4.7 k -10 V
-
ABC company leased new advanced computer equipment to STU Ltd on 1 January 2019.STULtd has to pay annual rental of $290,000 starting at 1 January 2019. It is a four years lease with ultimate rental...
-
A cycloid is the curve described by a point P on the circumference of a circular wheel of radius r rolling along the x axis. The curve is described in parametric form by the equations Use these...
-
Afence around a eld is shaped as shown in Figure P25. It consists of a rectangle of length L and width W and a right triangle that is symmetric about the central horizontal axis of the rectangle....
-
The four-sided gure shown in Figure P26 consists of two triangles having a common side a. The law of cosines for the top triangle states that a 2 = b 2 1 + c 2 1 - 2b 1 c 1 cos A 1 and a similar...
-
Growth Corps free cash flow is $1,000,000,000 and is expected to grow by at least 4% per year for the foreseeable future. They have $2,000,000,000 in long-term debt and 100,000,000 shares...
-
You are considering the purchase of an apartment complex that will generate net cash flows each of the next 20 years, starting at $400,000 in Year 1. You normally demand a 10% rate of return on such...
-
Jackson Company produces plastic that is used for injection molding applications such as gears for small motors, In 2016, the first year of operations, Jackson produced 4,200 tons of plastic and sold...

Study smarter with the SolutionInn App


