Question: In a continuous manufacturing process, chlorodifluoromethane (CHClF 2 ) initially at 10 bar and 420 K, passes through an adiabatic pressure reducing valve so that
In a continuous manufacturing process, chlorodifluoromethane (CHClF2) initially at 10 bar and 420 K, passes through an adiabatic pressure reducing valve so that its pressure is 0.1 bar (this last pressure is low enough that CHClF2 can be considered to be an ideal gas). At these operating conditions, the gas can be represented by a one-term virial equation of state:
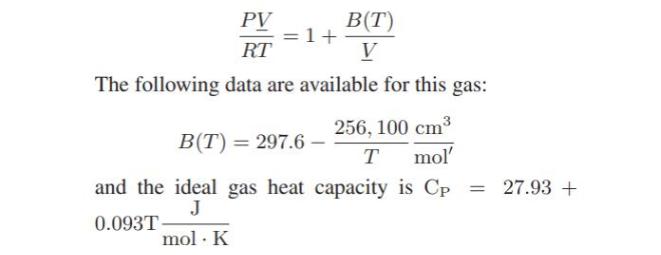
What is the temperature of the chlorodifluoromethane exiting valve? How much entropy is generated in the process per mole of chlorodifluoromethane that flows through the valve?
B(T) V PV RT The following data are available for this gas: 256, 100 cm T mol' B(T) = 297.6- and the ideal gas heat capacity is Cp = J 0.093T- mol. K = 27.93 +
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it
To determine the temperature of the chlorodifluoromethane exiting the valve and the entropy generated in the process we can follow these steps Step 1 ... View full answer

Get step-by-step solutions from verified subject matter experts


