Question: In a continuous manufacturing process, chlorodifluoromethane (CHClF 2 ), initially at 10 bar and 420 K, passes through an adiabatic pressure-reducing valve so that its
In a continuous manufacturing process, chlorodifluoromethane (CHClF2), initially at 10 bar and 420 K, passes through an adiabatic pressure-reducing valve so that its pressure drops to 0.1 bar (this last pressure low enough that CHClF2 can be considered to be an ideal gas). At these operating conditions, the gas can be represented by a one-term virial equation of state:
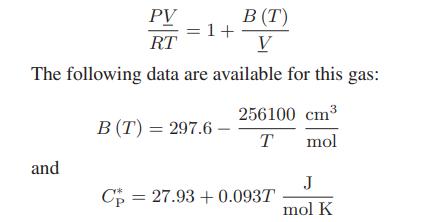
What is the temperature of the chlorodifluoromethane exiting the valve?
PV RT The following data are available for this gas: and = 1+ B (T) V B (T) = 297.6 - 256100 cm T mol Cp 27.93 +0.093T = J mol K
Step by Step Solution
3.39 Rating (149 Votes )
There are 3 Steps involved in it
Approach Since the pressurereducing valve is adiabatic there is no heat transfer into or out of the ... View full answer

Get step-by-step solutions from verified subject matter experts


