A gas is continuously passed through an adiabatic turbine at the rate of 2 mol/s. Its initial
Question:
A gas is continuously passed through an adiabatic turbine at the rate of 2 mol/s. Its initial temperature is 600 K, its initial pressure is 5 bar and its exiting pressure is 1 bar. Determine the maximum rate at which work can be obtained in this process. The gas is described by an augmented Clausius equation of state
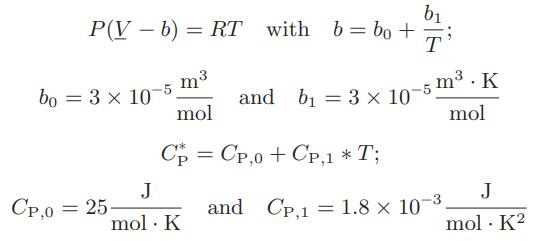
Transcribed Image Text:
b₁ P(V - b) = RT with b = bo+i T bo = 3 × 10-5 m3 mol Cp = Cp,o+Cp,1*T; CP,0 = 25 J mol. K and b₁ = 3 x 10-5 m³. K mol J mol. K² and CP,1 1.8 x 10-³. =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (3 reviews)
To determine the maximum rate at which work can be obtained from the gas in an adiabatic turbine we ...View the full answer

Answered By

Atuga Nichasius
I am a Highly skilled Online Tutor has a Bachelor’s Degree in Engineering as well as seven years of experience tutoring students in high school, bachelors and post graduate levels. I have a solid understanding of all learning styles as well as using asynchronous online platforms for tutoring needs. I individualise tutoring for students according to content tutoring needs assessments.
My strengths include good understanding of all teaching methods and learning styles and I am able to convey material to students in an easy to understand manner. I can also assists students with homework questions and test preparation strategies and I am able to help students in math, gre, business , and statistics
I consider myself to have excellent interpersonal and assessment skills with strong teaching presentation verbal and written communication
I love tutoring. I love doing it. I find it intrinsically satisfying to see the light come on in a student's eyes.
My first math lesson that I taught was when I was 5. My neighbor, still in diapers, kept skipping 4 when counting from 1 to 10. I worked with him until he could get all 10 numbers in a row, and match them up with his fingers.
My students drastically improve under my tutelage, generally seeing a two grade level improvement (F to C, C to A, for example), and all of them get a much clearer understanding!
I am committed to helping my students get the top grades no matter the cost. I will take extra hours with you, repeat myself a thousand times if I have to and guide you to the best of my ability until you understand the concept that I'm teaching you.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
A gas is continuously passed through an adiabatic turbine at the rate of 2 mol/s. Its initial temperature is 600 K, its initial pressure is 5 bar and its exiting pressure is 1 bar. Determine the...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
An ideal gas expands in an adiabatic turbine from 1200 K, 600 kPa to 700 K. Determine the turbine inlet volume flow rate of the gas, in m3/s, required to produce turbine work output at the rate of...
-
Song Corp's stock price at the end of last year was $26.25 and its earnings per share for the year were $1.30. What was its P/E ratio?
-
Ryan Corporation manufactures and sells a variety of household cleaning products in interstate commerce. On national television, Ryan falsely advertises that its laundry liquid is biodegradable. Has...
-
Describe three types of software that can assist in project quality management.
-
What is total quality management, and what are its objectives?
-
The following information is available for Fishel Manufacturing Company. Prepare the cost of goods manufactured schedule for the month ofApril. April 1 $10,000 5,000 April 30 $14,000 3,500 Raw...
-
The following data (dollar amounts in millions) are taken from the financial statements of Process 1 Industries Inc. (Click the icon to view the data.) Required Complete the following condensed...
-
A project for improving a billing process has the following precedence relationships and activity times: a. Draw the network diagram. b. Calculate the slack for each activity. Which activities are on...
-
Methane at 260 K is to be isothermally compressed from 0.1 MPa to 1.0 MPa. a. What is the minimum work required, and how much heat must be removed to keep the compression process isothermal? b. If...
-
In a continuous manufacturing process, chlorodifluoromethane (CHClF 2 ), initially at 10 bar and 420 K, passes through an adiabatic pressure-reducing valve so that its pressure drops to 0.1 bar (this...
-
Five balls move through the air as shown in Figure Q6.18. All five have the same size and shape. Air resistance is not negligible. Rank in order, from largest to smallest, the magnitudes of the...
-
Construct a confidence interval for p-P2 at the given level of confidence. x =26, n =229, x2 = 31, n = 302, 95% confidence The researchers are % confident the difference between the two population...
-
AP 9-2 (Moving Expenses) In May of the current year, following a dispute with her immediate superior, Ms. Elaine Fox resigned from her job in Halifax and began to look for other employment. She was...
-
Minimize the number of states in the following DFA: A b b a a a b b b E B a a
-
You have two dashboards in the same workspace named Production and Manufacturing. Your company's Power BI administrator creates the following two dashboard data classifications: Medium Impact (MEDI)...
-
Question 2: Red Rocks Corporation was organized on September 1. Red Rocks encountered the following events during the first month of operations. a. Received $65,000 cash from the investors who...
-
Inositols are hexahydroxycyclohexanes, with one hydroxyl group on each carbon atom of the ring. Although not strictly carbohydrates, they are obviously similar to pyranose sugars and do occur in...
-
Listed below are several terms and phrases associated with basic assumptions, broad accounting principles, and constraints. Pair each item from List A (by letter) with the item from List B that is...
-
Compute and plot the unit-step response of the following model. 10 + + 2 %3Df+ 7f
-
Find the reduced form of the following state model. -4 -1 2 -3 () 15
-
The following state model describes the motion of a certain mass connected to a spring, with viscous friction on the surface, where m = 1, c = 2, and k = 5. a. Use the initial function to plot the...
-
The country of Lebanon just invested $334,800 to build an amusement park. The amusement park is expected to produce cash inflows of $48,300 for 9 years and a cash inflow of $63,700 in Year 10. If...
-
Which of the following would not be shown in the operating activities section of the statement of cash flows? inventory sold collections from customers payments to suppliers exchanges of assets
-
If the month-end bank statement shows a balance of $75,000, outstanding checks are $54,000, a deposit of $15,000 was in transit at month end, and a check for $4,000 was erroneously charged by the...

Study smarter with the SolutionInn App


