Question: Redo Problem 13.10 using Aspen Plus. Problem 13.10 Hydrogen gas can be produced by the following reactions between propane and steam in the presence of
Redo Problem 13.10 using Aspen Plus.
Problem 13.10
Hydrogen gas can be produced by the following reactions between propane and steam in the presence of a nickel catalyst:
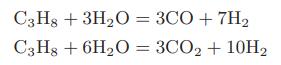
a. Compute the standard heat of reaction and the standard-state Gibbs energy change on reaction for each of the reactions at 1000 and 1100 K.
b. What is the equilibrium composition of the product gas at 1000 K and 1 bar if the inlet to the catalytic reactor is pure propane and steam in a 1-to-10 ratio?
c. Repeat calculation (b) at 1100 K
C3H8 + 3HO = 3CO + 7H C3H8 + 6HO = 3CO + 10H
Step by Step Solution
3.45 Rating (155 Votes )
There are 3 Steps involved in it
here is the solution to Problem 1310 using Aspen Plus a Standard heat of reaction and standardstate Gibbs energy change The standard heat of reaction ... View full answer

Get step-by-step solutions from verified subject matter experts


